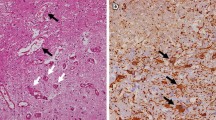
Abstract
Purpose of Review
Cerebral radiation necrosis (CRN) is a major dose-limiting adverse event of radiotherapy. The incidence rate of RN varies with the radiotherapy modality, total dose, dose fractionation, and the nature of the lesion being targeted. In addition to these known and controllable features, there is a stochastic component to the occurrence of CRN—the genetic profile of the host or the lesion and their role in the development of CRN.
Recent Findings
Recent studies provide some insight into the genetic mechanisms underlying radiation-induced brain injury. In addition to these incompletely understood host factors, the diagnostic criteria for CRN using structural and functional imaging are also not clear, though multiple structural and functional imaging modalities exist, a combination of which may prove to be the ideal diagnostic imaging approach. As the utilization of novel molecular therapies and immunotherapy increases, the incidence of CRN is expected to increase and its diagnosis will become more challenging. Tissue biopsies can be insensitive and suffer from sampling biases and procedural risks. Liquid biopsies represent a promising, accurate, and non-invasive diagnostic strategy, though this modality is currently in its infancy.
Summary
A better understanding of the pathogenesis of CRN will expand and optimize the diagnosis and management of CRN by better utilizing existing treatment options including bevacizumab, pentoxifylline, hyperbaric oxygen therapy, and laser interstitial thermal therapy.






Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
Chao ST, Ahluwalia MS, Barnett GH, Stevens GHJ, Murphy ES, Stockham AL, et al. Challenges with the diagnosis and treatment of cerebral radiation necrosis. Int J Radiat Oncol. 2013;87:449–57. https://doi.org/10.1016/j.ijrobp.2013.05.015.
Fischer AW, Holfelder H. Lokales Amyloid im Gehirn. Dtsch Zeitschrift Für Chir. 1930;227:475–83. https://doi.org/10.1007/BF02792795.
Sheline GE, Wara WM, Smith V. Therapeutic irradiation and brain injury. Int J Radiat Oncol Biol Phys. 1980;6:1215–28.
Rahmathulla G, Marko NF, Weil RJ. Cerebral radiation necrosis: a review of the pathobiology, diagnosis and management considerations. J Clin Neurosci. 2013;20:485–502. https://doi.org/10.1016/j.jocn.2012.09.011.
Gutin PH, Prados MD, Phillips TL, Wara WM, Larson DA, Leibel SA, et al. External irradiation followed by an interstitial high activity iodine-125 implant "boost" in the initial treatment of malignant gliomas: NCOG study 6G-82-2. Int J Radiat Oncol Biol Phys. 1991;21:601–6.
Shaw E, Arusell R, Scheithauer B, O’Fallon J, O’Neill B, Dinapoli R, et al. Prospective randomized trial of low- versus high-dose radiation therapy in adults with Supratentorial low-grade glioma: initial report of a north central cancer treatment group/radiation therapy oncology group/eastern cooperative oncology group study. J Clin Oncol. 2002;20:2267–76. https://doi.org/10.1200/JCO.2002.09.126.
Corn BW, Yousem DM, Scott CB, Rotman M, Asbell SO, Nelson DF, et al. White matter changes are correlated significantly with radiation dose. Observations from a randomized dose-escalation trial for malignant glioma (radiation therapy oncology group 83-02). Cancer. 1994;74:2828–35. https://doi.org/10.1002/1097-0142(19941115)74:10<2828::AID-CNCR2820741014>3.0.CO;2-K.
Fetcko K, Lukas RV, Watson GA, Zhang L, Dey M. Survival and complications of stereotactic radiosurgery. Medicine (Baltimore). 2017;96:e8293. https://doi.org/10.1097/MD.0000000000008293.
Cabrera AR, Cuneo KC, Desjardins A, Sampson JH, McSherry F, Herndon JE, et al. Concurrent stereotactic radiosurgery and bevacizumab in recurrent malignant gliomas: a prospective trial. Int J Radiat Oncol. 2013;86:873–9. https://doi.org/10.1016/j.ijrobp.2013.04.029.
Gerosa M, Nicolato A, Foroni R, Zanotti B, Tomazzoli L, Miscusi M, et al. Gamma knife radiosurgery for brain metastases: a primary therapeutic option. J Neurosurg. 2002;97(5 Suppl):515–24.
Petrovich Z, Yu C, Giannotta SL, O’Day S, Apuzzo ML. Survival and pattern of failure in brain metastasis treated with stereotactic gamma knife radiosurgery. J Neurosurg. 2002;97(5 Suppl):499–506.
Kim JW, Park HR, Lee JM, Kim JW, Chung H-T, Kim DG, et al. Fractionated stereotactic gamma knife radiosurgery for large brain metastases: a retrospective, Single Center Study. PLoS One. 2016;11:e0163304. https://doi.org/10.1371/journal.pone.0163304.
Koffer P, Chan J, Rava P, Gorovets D, Ebner D, Savir G, et al. Repeat stereotactic radiosurgery for locally recurrent brain metastases. World Neurosurg. 2017;104:589–93. https://doi.org/10.1016/j.wneu.2017.04.103.
Jeong WJ, Park JH, Lee EJ, Kim JH, Kim CJ, Cho YH. Efficacy and safety of fractionated stereotactic radiosurgery for large brain metastases. J Korean Neurosurg Soc. 2015;58:217–24. https://doi.org/10.3340/jkns.2015.58.3.217.
Prabhu RS, Press RH, Patel KR, Boselli DM, Symanowski JT, Lankford SP, et al. Single-fraction stereotactic radiosurgery (SRS) alone versus surgical resection and SRS for large brain metastases: a multi-institutional analysis. Int J Radiat Oncol. 2017;99:459–67. https://doi.org/10.1016/j.ijrobp.2017.04.006.
Minniti G, Scaringi C, Paolini S, Lanzetta G, Romano A, Cicone F, et al. Single-fraction versus multifraction (3 × 9 Gy) stereotactic radiosurgery for large (>2 cm) brain metastases: a comparative analysis of local control and risk of radiation-induced brain necrosis. Int J Radiat Oncol. 2016;95:1142–8. https://doi.org/10.1016/j.ijrobp.2016.03.013.
Narloch JL, Farber SH, Sammons S, McSherry F, Herndon JE, Hoang JK, et al. Biopsy of enlarging lesions after stereotactic radiosurgery for brain metastases frequently reveals radiation necrosis. Neuro-Oncology. 2017;19:1391–7. https://doi.org/10.1093/neuonc/nox090.
Kim JM, Miller JA, Kotecha R, Xiao R, Juloori A, Ward MC, et al. The risk of radiation necrosis following stereotactic radiosurgery with concurrent systemic therapies. J Neuro-Oncol. 2017;133:357–68. https://doi.org/10.1007/s11060-017-2442-8.
Lehrer EJ, Peterson J, Brown PD, Sheehan JP, Quiñones-Hinojosa A, Zaorsky NG, et al. Treatment of brain metastases with stereotactic radiosurgery and immune checkpoint inhibitors: an international meta-analysis of individual patient data. Radiother Oncol. 2018;130:104–12. https://doi.org/10.1016/j.radonc.2018.08.025.
Lawrence YR, Li XA, el Naqa I, Hahn CA, Marks LB, Merchant TE, et al. Radiation dose–volume effects in the brain. Int J Radiat Oncol. 2010;76:S20–7. https://doi.org/10.1016/j.ijrobp.2009.02.091.
Voges J, Treuer H, Lehrke R, Kocher M, Staar S, Müller RP, et al. Risk analysis of LINAC radiosurgery in patients with arteriovenous malformation (AVM). Acta Neurochir Suppl. 1997;68:118–23.
Flickinger JC, Lunsford LD, Kondziolka D, Maitz AH, Epstein AH, Simons SR, et al. Radiosurgery and brain tolerance: an analysis of neurodiagnostic imaging changes after gamma knife radiosurgery for arteriovenous malformations. Int J Radiat Oncol Biol Phys. 1992;23:19–26.
Abdulla S, Saada J, Johnson G, Jefferies S, Ajithkumar T. Tumour progression or pseudoprogression? A review of post-treatment radiological appearances of glioblastoma. Clin Radiol. 2015;70:1299–312. https://doi.org/10.1016/j.crad.2015.06.096.
Kolesnick R, Fuks Z. Radiation and ceramide-induced apoptosis. Oncogene. 2003;22:5897–906. https://doi.org/10.1038/sj.onc.1206702.
Remler MP, Marcussen WH, Tiller-Borsich J. The late effects of radiation on the blood brain barrier. Int J Radiat Oncol Biol Phys. 1986;12:1965–9.
Fajardo LF, Berthrong M. Vascular lesions following radiation. Pathol Annu. 1988;23(Pt 1):297–330.
Fike JR, Rosi S, Limoli CL. Neural precursor cells and central nervous system radiation sensitivity. Semin Radiat Oncol. 2009;19:122–32. https://doi.org/10.1016/j.semradonc.2008.12.003.
Kudo S, Suzuki Y, Noda S-E, Mizui T, Shirai K, Okamoto M, et al. Comparison of the radiosensitivities of neurons and glial cells derived from the same rat brain. Exp Ther Med. 2014;8:754–8. https://doi.org/10.3892/etm.2014.1802.
Nordal RA, Nagy A, Pintilie M, Wong CS. Hypoxia and hypoxia-inducible factor-1 target genes in central nervous system radiation injury: a role for vascular endothelial growth factor. Clin Cancer Res. 2004;10:3342–53. https://doi.org/10.1158/1078-0432.CCR-03-0426.
Yoritsune E, Furuse M, Kuwabara H, Miyata T, Nonoguchi N, Kawabata S, et al. Inflammation as well as angiogenesis may participate in the pathophysiology of brain radiation necrosis. J Radiat Res. 2014;55:803–11. https://doi.org/10.1093/jrr/rru017.
Yang R, Duan C, Yuan L, Engelbach JA, Tsien CI, Beeman SC, et al. Inhibitors of HIF-1α and CXCR4 mitigate the development of radiation necrosis in mouse brain. Int J Radiat Oncol. 2018;100:1016–25. https://doi.org/10.1016/j.ijrobp.2017.12.257.
Woodworth GF, Garzon-Muvdi T, Ye X, Blakeley JO, Weingart JD, Burger PC. Histopathological correlates with survival in reoperated glioblastomas. J Neuro-Oncol. 2013;113:485–93. https://doi.org/10.1007/s11060-013-1141-3.
Tihan T, Barletta J, Parney I, Lamborn K, Sneed PK, Chang S. Prognostic value of detecting recurrent glioblastoma multiforme in surgical specimens from patients after radiotherapy: should pathology evaluation alter treatment decisions? Hum Pathol. 2006;37:272–82. https://doi.org/10.1016/j.humpath.2005.11.010.
McGirt MJ, Bulsara KR, Cummings TJ, New KC, Little KM, Friedman HS, et al. Prognostic value of magnetic resonance imaging—guided stereotactic biopsy in the evaluation of recurrent malignant astrocytoma compared with a lesion due to radiation effect. J Neurosurg. 2003;98:14–20. https://doi.org/10.3171/jns.2003.98.1.0014.
Rusthoven KE, Olsen C, Franklin W, Kleinschmidt-DeMasters BK, Kavanagh BD, Gaspar LE, et al. Favorable prognosis in patients with high-grade glioma with radiation necrosis: the University of Colorado Reoperation Series. Int J Radiat Oncol. 2011;81:211–7. https://doi.org/10.1016/j.ijrobp.2010.04.069.
Ali FS, Hussain MR, Gutiérrez C, Demireva P, Ballester LY, Zhu J-J, et al. Cognitive disability in adult patients with brain tumors. Cancer Treat Rev. 2018;65:33–40. https://doi.org/10.1016/J.CTRV.2018.02.007.
Takenaka N, Imanishi T, Sasaki H, Shimazaki K, Sugiura H, Kitagawa Y, et al. Delayed radiation necrosis with extensive brain edema after gamma knife radiosurgery for multiple cerebral cavernous malformations-case report. Neurol Med Chir (Tokyo). 2003;43:391–5. https://doi.org/10.2176/nmc.43.391.
Woo E, Chan Y-F, Lam K, Lok ASF, Yu Y-L, Huang C-Y. Apoplectic intracerebral hemorrhage: an unusual complication of cerebral radiation necrosis. Pathology. 1987;19:95–8. https://doi.org/10.3109/00313028709065146.
Ruben JD, Dally M, Bailey M, Smith R, McLean CA, Fedele P. Cerebral radiation necrosis: incidence, outcomes, and risk factors with emphasis on radiation parameters and chemotherapy. Int J Radiat Oncol Biol Phys. 2006;65:499–508. https://doi.org/10.1016/j.ijrobp.2005.12.002.
Korytko T, Radivoyevitch T, Colussi V, Wessels BW, Pillai K, Maciunas RJ, et al. 12 Gy gamma knife radiosurgical volume is a predictor for radiation necrosis in non-AVM intracranial tumors. Int J Radiat Oncol. 2006;64:419–24. https://doi.org/10.1016/j.ijrobp.2005.07.980.
Mayer R, Sminia P. Reirradiation tolerance of the human brain. Int J Radiat Oncol. 2008;70:1350–60. https://doi.org/10.1016/j.ijrobp.2007.08.015.
Fogh SE, Andrews DW, Glass J, Curran W, Glass C, Champ C, et al. Hypofractionated stereotactic radiation therapy: an effective therapy for recurrent high-grade gliomas. J Clin Oncol. 2010;28:3048–53. https://doi.org/10.1200/JCO.2009.25.6941.
Blonigen BJ, Steinmetz RD, Levin L, Lamba MA, Warnick RE, Breneman JC. Irradiated volume as a predictor of brain radionecrosis after linear accelerator stereotactic radiosurgery. Int J Radiat Oncol Biol Phys. 2010;77:996–1001. https://doi.org/10.1016/j.ijrobp.2009.06.006.
Minniti G, Clarke E, Lanzetta G, Osti M, Trasimeni G, Bozzao A, et al. Stereotactic radiosurgery for brain metastases: analysis of outcome and risk of brain radionecrosis. Radiat Oncol. 2011;6:48. https://doi.org/10.1186/1748-717X-6-48.
Marks JE, Baglan RJ, Prassad SC, Blank WF. Cerebral radionecrosis: incidence and risk in relation to dose, time, fractionation and volume. Int J Radiat Oncol Biol Phys. 1981;7:243–52.
Dohm A, McTyre ER, Okoukoni C, Henson A, Cramer CK, LeCompte MC, et al. Staged stereotactic radiosurgery for large brain metastases: local control and clinical outcomes of a one-two punch technique. Neurosurgery. 2018;83:114–21. https://doi.org/10.1093/neuros/nyx355.
Patel KR, Chowdhary M, Switchenko JM, Kudchadkar R, Lawson DH, Cassidy RJ, et al. BRAF inhibitor and stereotactic radiosurgery is associated with an increased risk of radiation necrosis. Melanoma Res. 2016;26:387–94. https://doi.org/10.1097/CMR.0000000000000268.
Diao K, Bian SX, Routman DM, Yu C, Ye JC, Wagle NA, et al. Stereotactic radiosurgery and ipilimumab for patients with melanoma brain metastases: clinical outcomes and toxicity. J Neuro-Oncol. 2018;139:421–9. https://doi.org/10.1007/s11060-018-2880-y.
Colaco RJ, Martin P, Kluger HM, Yu JB, Chiang VL. Does immunotherapy increase the rate of radiation necrosis after radiosurgical treatment of brain metastases? J Neurosurg. 2016;125:17–23. https://doi.org/10.3171/2015.6.JNS142763.
• Miller JA, Bennett EE, Xiao R, Kotecha R, Chao ST, Vogelbaum MA, et al. Association between radiation necrosis and tumor biology after stereotactic radiosurgery for brain metastasis. Int J Radiat Oncol. 2016;96:1060–9. https://doi.org/10.1016/j.ijrobp.2016.08.039. Highlights the impact of tumor biology on the development of radiation necrosis.
Li H, Li J, Cheng G, Zhang J, Li X. IDH mutation and MGMT promoter methylation are associated with the pseudoprogression and improved prognosis of glioblastoma multiforme patients who have undergone concurrent and adjuvant temozolomide-based chemoradiotherapy. Clin Neurol Neurosurg. 2016;151:31–6. https://doi.org/10.1016/j.clineuro.2016.10.004.
Brandes AA, Franceschi E, Tosoni A, Blatt V, Pession A, Tallini G, et al. MGMT promoter methylation status can predict the incidence and outcome of pseudoprogression after concomitant Radiochemotherapy in newly diagnosed glioblastoma patients. J Clin Oncol. 2008;26:2192–7. https://doi.org/10.1200/JCO.2007.14.8163.
Roberts SA, Spreadborough AR, Bulman B, Barber JBP, Evans DGR, Scott D. Heritability of cellular radiosensitivity: a marker of low-penetrance predisposition genes in breast cancer? Am J Hum Genet. 1999;65:784–94. https://doi.org/10.1086/302544.
Gatti RA. The inherited basis of human radiosensitivity. Acta Oncol. 2001;40:702–11.
Alter BP. Radiosensitivity in Fanconi’s anemia patients. Radiother Oncol. 2002;62:345–7.
Taylor AMR, Harnden DG, Arlett CF, Harcourt SA, Lehmann AR, Stevens S, et al. Ataxia telangiectasia: a human mutation with abnormal radiation sensitivity. Nature. 1975;258:427–9. https://doi.org/10.1038/258427a0.
• Wang T-M, Shen G-P, Chen M-Y, Zhang J-B, Sun Y, He J, et al. Genome-wide association study of susceptibility loci for radiation-induced brain injury. J Natl Cancer Inst. 2018. https://doi.org/10.1093/jnci/djy150. First study to identify an SNP that impacts radiosensitivity and may increase the risk of radiation necrosis.
Siu A, Wind JJ, Iorgulescu JB, Chan TA, Yamada Y, Sherman JH. Radiation necrosis following treatment of high grade glioma—a review of the literature and current understanding. Acta Neurochir. 2012;154:191–201. https://doi.org/10.1007/s00701-011-1228-6.
GDC-0449 in Treating patients with recurrent glioblastoma multiforme that can be removed by surgery - Tabular View - ClinicalTrials.gov. n.d.
Verma N, Cowperthwaite MC, Burnett MG, Markey MK. Differentiating tumor recurrence from treatment necrosis: a review of neuro-oncologic imaging strategies. Neuro-Oncology. 2013;15:515–34. https://doi.org/10.1093/neuonc/nos307.
Zhuang H, Zheng Y, Wang J, Chang JY, Wang X, Yuan Z, et al. Analysis of risk and predictors of brain radiation necrosis after radiosurgery. Oncotarget. 2016;7:7773. https://doi.org/10.18632/ONCOTARGET.6532.
Dequesada IM, Quisling RG, Yachnis A, Friedman WA. Can standard magnetic resonance imaging reliably distinguish recurrent tumor from radiation necrosis after radiosurgery for brain metastases? A radiographic-pathological study. Neurosurgery. 2008;63:898–904. https://doi.org/10.1227/01.NEU.0000333263.31870.31.
Sundgren PC, Fan X, Weybright P, Welsh RC, Carlos RC, Petrou M, et al. Differentiation of recurrent brain tumor versus radiation injury using diffusion tensor imaging in patients with new contrast-enhancing lesions. Magn Reson Imaging. 2006;24:1131–42. https://doi.org/10.1016/j.mri.2006.07.008.
Sinha S, Bastin ME, Whittle IR, Wardlaw JM. Diffusion tensor MR imaging of high-grade cerebral gliomas. AJNR Am J Neuroradiol. 2002;23:520–7.
Rock JP, Scarpace L, Hearshen D, Gutierrez J, Fisher JL, Rosenblum M, et al. Associations among magnetic resonance spectroscopy, apparent diffusion coefficients, and image-guided histopathology with special attention to radiation necrosis. Neurosurgery. 2004;54:1111–7 discussion 1117-9.
Hein PA, Eskey CJ, Dunn JF, Hug EB. Diffusion-weighted imaging in the follow-up of treated high-grade gliomas: tumor recurrence versus radiation injury. AJNR Am J Neuroradiol. 2004;25:201–9.
Nazem-Zadeh M-R, Chapman CH, Chenevert T, Lawrence TS, Ten Haken RK, Tsien CI, et al. Response-driven imaging biomarkers for predicting radiation necrosis of the brain. Phys Med Biol. 2014;59:2535–47. https://doi.org/10.1088/0031-9155/59/10/2535.
Mitsuya K, Nakasu Y, Horiguchi S, Harada H, Nishimura T, Bando E, et al. Perfusion weighted magnetic resonance imaging to distinguish the recurrence of metastatic brain tumors from radiation necrosis after stereotactic radiosurgery. J Neuro-Oncol. 2010;99:81–8. https://doi.org/10.1007/s11060-009-0106-z.
Kim YH, Oh SW, Lim YJ, Park C-K, Lee S-H, Kang KW, et al. Differentiating radiation necrosis from tumor recurrence in high-grade gliomas: assessing the efficacy of 18F-FDG PET, 11C-methionine PET and perfusion MRI. Clin Neurol Neurosurg. 2010;112:758–65. https://doi.org/10.1016/j.clineuro.2010.06.005.
Birken DL, Oldendorf WH. N-acetyl-L-aspartic acid: a literature review of a compound prominent in 1H-NMR spectroscopic studies of brain. Neurosci Biobehav Rev. 1989;13:23–31.
Degaonkar MN, Pomper MG, Barker PB. Quantitative proton magnetic resonance spectroscopic imaging: regional variations in the corpus callosum and cortical gray matter. J Magn Reson Imaging. 2005;22:175–9. https://doi.org/10.1002/jmri.20353.
Chan YL, Yeung DK, Leung SF, Cao G. Proton magnetic resonance spectroscopy of late delayed radiation-induced injury of the brain. J Magn Reson Imaging. 1999;10:130–7.
Parvez K, Parvez A, Zadeh G. The diagnosis and treatment of pseudoprogression, radiation necrosis and brain tumor recurrence. Int J Mol Sci. 2014;15:11832–46. https://doi.org/10.3390/ijms150711832.
Blanchet L, Krooshof PWT, Postma GJ, Idema AJ, Goraj B, Heerschap A, et al. Discrimination between metastasis and glioblastoma multiforme based on morphometric analysis of MR images. Am J Neuroradiol. 2011;32:67–73. https://doi.org/10.3174/ajnr.A2269.
Manjila S, Knudson KE, Johnson C, Sloan AE. Monteris AXiiiS stereotactic miniframe for intracranial biopsy. Neurosurgery. 2015;12:1. https://doi.org/10.1227/NEU.0000000000001124.
• Koch CJ, Lustig RA, Yang X-Y, Jenkins WT, Wolf RL, Martinez-Lage M, et al. Microvesicles as a biomarker for tumor progression versus treatment effect in radiation/temozolomide-treated glioblastoma patients. Transl Oncol. 2014;7:752–8. https://doi.org/10.1016/j.tranon.2014.10.004. Preliminary study on the utility of liquid biopsy in diagnosing radiation necrosis.
Soler DC, Young AB, Cooper KD, Kerstetter-Fogle A, Barnholtz-Sloan JS, Gittleman H, et al. The ratio of HLA-DR and VNN2+ expression on CD14+ myeloid derived suppressor cells can distinguish glioblastoma from radiation necrosis patients. J Neuro-Oncol. 2017;134:189–96. https://doi.org/10.1007/s11060-017-2508-7.
Ballester LY, Lu G, Zorofchian S, Vantaku V, Putluri V, Yan Y, et al. Analysis of cerebrospinal fluid metabolites in patients with primary or metastatic central nervous system tumors. Acta Neuropathol Commun. 2018;6:85. https://doi.org/10.1186/s40478-018-0588-z.
Zorofchian S, Iqbal F, Rao M, Aung PP, Esquenazi Y, Ballester LY. Circulating tumour DNA, microRNA and metabolites in cerebrospinal fluid as biomarkers for central nervous system malignancies. J Clin Pathol. 2019;72:271–80. https://doi.org/10.1136/jclinpath-2018-205414.
Zorofchian S, Lu G, Zhu J-J, Duose DY, Windham J, Esquenazi Y, et al. Detection of the MYD88 p.L265P mutation in the CSF of a patient with secondary central nervous system lymphoma. Front Oncol. 2018;8:382. https://doi.org/10.3389/fonc.2018.00382.
Miller AM, Shah RH, Pentsova EI, Pourmaleki M, Briggs S, Distefano N, et al. Tracking tumour evolution in glioma through liquid biopsies of cerebrospinal fluid. Nature. 2019;565:654–8. https://doi.org/10.1038/s41586-019-0882-3.
Sneed PK, Mendez J, Vemer-van den Hoek JGM, Seymour ZA, Ma L, Molinaro AM, et al. Adverse radiation effect after stereotactic radiosurgery for brain metastases: incidence, time course, and risk factors. J Neurosurg. 2015;123:373–86. https://doi.org/10.3171/2014.10.JNS141610.
Han J, Thompson P, Beutler B. Dexamethasone and pentoxifylline inhibit endotoxin-induced cachectin/tumor necrosis factor synthesis at separate points in the signaling pathway. J Exp Med. 1990;172:391–4.
Hong JH, Chiang CS, Sun JR, Withers HR, McBride WH. Induction of c-fos and junB mRNA following in vivo brain irradiation. Brain Res Mol Brain Res. 1997;48:223–8.
Wang XS, Ying HM, He XY, Zhou ZR, Wu YR, Hu CS. Treatment of cerebral radiation necrosis with nerve growth factor: a prospective, randomized, controlled phase II study. Radiother Oncol. 2016;120:69–75. https://doi.org/10.1016/j.radonc.2016.04.027.
Glantz MJ, Burger PC, Friedman AH, Radtke RA, Massey EW, Schold SC. Treatment of radiation-induced nervous system injury with heparin and warfarin. Neurology. 1994;44:2020–7.
Williamson R, Kondziolka D, Kanaan H, Lunsford LD, Flickinger JC. Adverse radiation effects after radiosurgery may benefit from oral vitamin E and pentoxifylline therapy: a pilot study. Stereotact Funct Neurosurg. 2008;86:359–66. https://doi.org/10.1159/000163557.
George C, Hart B, Robert C, Thompson E. The treatment of cerebral ischemia with hyperbaric oxygen (OHP) the treatment of cerebral ischemia with hyperbaric oxygen (OHP). Stroke.1971;2:247–50. https://doi.org/10.1161/01.STR.2.3.247.
Woo E, Lam K, Yu YL, Lee WH, Huang CY. Cerebral radionecrosis: is surgery necessary? J Neurol Neurosurg Psychiatry. 1987;50:1407–14. https://doi.org/10.1136/jnnp.50.11.1407.
Sharma M, Balasubramanian S, Silva D, Barnett GH, Mohammadi AM. Laser interstitial thermal therapy in the management of brain metastasis and radiation necrosis after radiosurgery: an overview. Expert Rev Neurother. 2016;16:223–32. https://doi.org/10.1586/14737175.2016.1135736.
Rahmathulla G, Recinos PF, Valerio JE, Chao S, Barnett GH. Laser interstitial thermal therapy for focal cerebral radiation necrosis: a case report and literature review. Stereotact Funct Neurosurg. 2012;90:192–200. https://doi.org/10.1159/000338251.
Levin VA, Bidaut L, Hou P, Kumar AJ, Wefel JS, Bekele BN, et al. Randomized double-blind placebo-controlled trial of bevacizumab therapy for radiation necrosis of the central nervous system. Int J Radiat Oncol. 2011;79:1487–95. https://doi.org/10.1016/j.ijrobp.2009.12.061.
Delishaj D, Ursino S, Pasqualetti F, Cristaudo A, Cosottini M, Fabrini MG, et al. Bevacizumab for the treatment of radiation-induced cerebral necrosis: a systematic review of the literature. J Clin Med Res. 2017;9:273–80. https://doi.org/10.14740/jocmr2936e.
Low-dose intra-arterial bevacizumab for edema and radiation necrosis therapeutic intervention (LIBERTI) - Full Text View - ClinicalTrials.gov. n.d.
Ozturk B, Egehan I, Atavci S, Kitapci M. Pentoxifylline in prevention of radiation-induced lung toxicity in patients with breast and lung cancer: a double-blind randomized trial. Int J Radiat Oncol Biol Phys. 2004;58:213–9.
Dion MW, Hussey DH, Doornbos JF, Vigliotti AP, Wen BC, Anderson B. Preliminary results of a pilot study of pentoxifylline in the treatment of late radiation soft tissue necrosis. Int J Radiat Oncol Biol Phys. 1990;19:401–7.
Evaluation of the use of trental and vitamin E For prophylaxis of radiation necrosis - Tabular View - ClinicalTrials.gov. n.d.
Ohguri T, Imada H, Kohshi K, Kakeda S, Ohnari N, Morioka T, et al. Effect of prophylactic hyperbaric oxygen treatment for radiation-induced brain injury after stereotactic radiosurgery of brain metastases. Int J Radiat Oncol. 2007;67:248–55. https://doi.org/10.1016/j.ijrobp.2006.08.009.
Cihan YB, Uzun G, Yildiz S, Dönmez H. Hyperbaric oxygen therapy for radiation-induced brain necrosis in a patient with primary central nervous system lymphoma. J Surg Oncol. 2009;100:732–5. https://doi.org/10.1002/jso.21387.
Alyahya M, Mittal S, Rowe D, Machtay M, Ali SJ, Selman W, et al. RTHP-07. Treatment of brain radiation necrosis with hyperbaric oxygen: report of 6 cases. Neuro Oncol. 2017;19:vi220. https://doi.org/10.1093/neuonc/nox168.892.
Mou Y, Sai K, Wang Z, Zhang X, Lu Y, Wei D, et al. Surgical management of radiation-induced temporal lobe necrosis in patients with nasopharyngeal carcinoma: report of 14 cases. Head Neck. 2011;33:1493–500. https://doi.org/10.1002/hed.21639.
Esquenazi Y, Kalamangalam GP, Slater JD, Knowlton RC, Friedman E, Morris S-A, et al. Stereotactic laser ablation of epileptogenic periventricular nodular heterotopia. Epilepsy Res. 2014;108:547–54. https://doi.org/10.1016/j.eplepsyres.2014.01.009.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Neuro-oncology
Electronic Supplementary Material
Supplementary Table 1
(DOCX 15 kb)
Rights and permissions
About this article
Cite this article
Ali, F.S., Arevalo, O., Zorofchian, S. et al. Cerebral Radiation Necrosis: Incidence, Pathogenesis, Diagnostic Challenges, and Future Opportunities. Curr Oncol Rep 21, 66 (2019). https://doi.org/10.1007/s11912-019-0818-y
Published:
DOI: https://doi.org/10.1007/s11912-019-0818-y