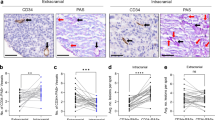
Abstract
Delayed radiation necrosis is a well-known adverse event following radiotherapy for brain diseases and has been studied since the 1930s. The primary pathogenesis is thought to be the direct damage to endothelial and glial cells, particularly oligodendrocytes, which causes vascular hyalinization and demyelination. This primary pathology leads to tissue inflammation and ischemia, inducing various tissue protective responses including angiogenesis. Macrophages and lymphocytes then infiltrate the surrounding areas of necrosis, releasing inflammatory cytokines such as interleukin (IL)-1α, IL-6, and tumor necrosis factor (TNF)-α. Microglia also express these inflammatory cytokines. Reactive astrocytes play an important role in angiogenesis, expressing vascular endothelial growth factor (VEGF). Some chemokine networks, like the CXCL12/CXCR4 axis, are upregulated by tissue inflammation. Hypoxia may mediate the cell–cell interactions among reactive astrocytes, macrophages, and microglial cells around the necrotic core. Recently, bevacizumab, an anti-VEGF antibody, has demonstrated promising results as an alternative treatment for radiation necrosis. The importance of VEGF in the pathophysiology of brain radiation necrosis is being recognized. The discovery of new molecular targets could facilitate novel treatments for radiation necrosis. This literature review will focus on recent work characterizing delayed radiation necrosis in the brain.



Similar content being viewed by others
References
Schultheiss TE, Kun LE, Ang KK, Stephens LC (1995) Radiation response of the central nervous system. Int J Radiat Oncol Biol Phys 31(5):1093–1112
Valk PE, Dillon WP (1991) Radiation injury of the brain. AJNR Am J Neuroradiol 12(1):45–62
Fischer AW, Holfelder H (1930) Lokales amyloid in gehirn. Dtsch Z Chir 227:475–483
Arnold A, Bailey P, Laughlin JS (1954) Effects of beatron radiations on the brain of primates. Neurology 4(3):165–178
Ruben JD, Dally M, Bailey M, Smith R, McLean CA, Fedele P (2006) Cerebral radiation necrosis: incidence, outcomes, and risk factors with emphasis on radiation parameters and chemotherapy. Int J Radiat Oncol Biol Phys 65(2):499–508
Jagannathan J, Petit JH, Balsara K, Hudes R, Chin LS (2004) Long-term survival after gamma knife radiosurgery for primary and metastatic brain tumors. Am J Clin Oncol 27(5):441–444
Iuchi T, Hatano K, Narita Y, Kodama T, Yamaki T, Osato K (2006) Hypofractionated high-dose irradiation for the treatment of malignant astrocytomas using simultaneous integrated boost technique by IMRT. Int J Radiat Oncol Biol Phys 64(5):1317–1324
Kawabata S, Miyatake S, Kuroiwa T, Yokoyama K, Doi A, Iida K, Miyata S, Nonoguchi N, Michiue H, Takahashi M, Inomata T, Imahori Y, Kirihata M, Sakurai Y, Maruhashi A, Kumada H, Ono K (2009) Boron neutron capture therapy for newly diagnosed glioblastoma. J Radiat Res 50(1):51–60
Mizumoto M, Tsuboi K, Igaki H, Yamamoto T, Takano S, Oshiro Y, Hayashi Y, Hashii H, Kanemoto A, Nakayama H, Sugahara S, Sakurai H, Matsumura A, Tokuuye K (2010) Phase I/II trial of hyperfractionated concomitant boost proton radiotherapy for supratentorial glioblastoma multiforme. Int J Radiat Oncol Biol Phys 77(1):98–105
Brandes AA, Franceschi E, Tosoni A, Blatt V, Pession A, Tallini G, Bertorelle R, Bartolini S, Calbucci F, Andreoli A, Frezza G, Leonardi M, Spagnolli F, Ermani M (2008) MGMT promoter methylation status can predict the incidence and outcome of pseudoprogression after concomitant radiochemotherapy in newly diagnosed glioblastoma patients. J Clin Oncol 26(13):2192–2197
Brandsma D, Stalpers L, Taal W, Sminia P, van den Bent MJ (2008) Clinical features, mechanisms, and management of pseudoprogression in malignant gliomas. Lancet Oncol 9(5):453–461
Tsao MN, Li YQ, Lu G, Xu Y, Wong CS (1999) Upregulation of vascular endothelial growth factor is associated with radiation-induced blood-spinal cord barrier breakdown. J Neuropathol Exp Neurol 58(10):1051–1060
Li YQ, Ballinger JR, Nordal RA, Su ZF, Wong CS (2001) Hypoxia in radiation-induced blood-spinal cord barrier breakdown. Cancer Res 61(8):3348–3354
Nordal RA, Nagy A, Pintilie M, Wong CS (2004) Hypoxia and hypoxia-inducible factor-1 target genes in central nervous system radiation injury: a role for vascular endothelial growth factor. Clin Cancer Res 10(10):3342–3353
Nonoguchi N, Miyatake S, Fukumoto M, Furuse M, Hiramatsu R, Kawabata S, Kuroiwa T, Tsuji M, Ono K (2011) The distribution of vascular endothelial growth factor-producing cells in clinical radiation necrosis of the brain: pathological consideration of their potential roles. J Neurooncol 105(2):423–431
Furuse M, Nonoguchi N, Kawabata S, Miyata T, Toho T, Kuroiwa T, Miyatake S (2015) Intratumoral and peritumoral post-irradiation changes, but not viable tumor tissue, may respond to bevacizumab in previously irradiated meningiomas. Radiat Oncol 10:156
Furuse M, Kawabata S, Kuroiwa T, Miyatake S (2011) Repeated treatments with bevacizumab for recurrent radiation necrosis in patients with malignant brain tumors: a report of 2 cases. J Neurooncol 102(3):471–475
Furuse M, Nonoguchi N, Kawabata S, Yoritsune E, Takahashi M, Inomata T, Kuroiwa T, Miyatake S (2013) Bevacizumab treatment for symptomatic radiation necrosis diagnosed by amino acid PET. Jpn J Clin Oncol 43(3):337–341
Miyatake S, Nonoguchi N, Furuse M, Yoritsune E, Miyata T, Kawabata S, Kuroiwa T (2015) Pathophysiology, diagnosis, and treatment of radiation necrosis in the brain. Neurol Med Chir 55(Suppl 1):50–59
Pennybacker J, Russell DS (1948) Necrosis of the brain due to radiation therapy; clinical and pathological observations. J Neurol Neurosurg Psychiatry 11(3):183–198
Russell DS, Wilson CW, Tansley K (1949) Experimental radio-necrosis of the brain in rabbits. J Neurol Neurosurg Psychiatry 12(3):187–195
McDonald LW, Hayes TL (1967) The role of capillaries in the pathogenesis of delayed radionecrosis of brain. Am J Pathol 50(5):745–764
Martins AN, Johnston JS, Henry JM, Stoffel TJ, Di Chiro G (1977) Delayed radiation necrosis of the brain. J Neurosurg 47(3):336–345
O’Connor MM, Mayberg MR (2000) Effects of radiation on cerebral vasculature: a review. Neurosurgery 46(1):138–149
Kondziolka D, Lunsford LD, Claassen D, Maitz AH, Flickinger JC (1992) Radiobiology of radiosurgery: part I. The normal rat brain model. Neurosurgery 31(2):271–279
Yoritsune E, Furuse M, Kuwabara H, Miyata T, Nonoguchi N, Kawabata S, Hayasaki H, Kuroiwa T, Ono K, Shibayama Y, Miyatake S (2014) Inflammation as well as angiogenesis may participate in the pathophysiology of brain radiation necrosis. J Radiat Res 55(4):803–811
Mastaglia FL, McDonald WI, Watson JV, Yogendran K (1976) Effects of x-radiation on the spinal cord: an experimental study of the morphological changes in central nerve fibres. Brain 99(1):101–122
Arnold A, Bailey P, Harvey RA, Haas LL, Laughlin JS (1954) Changes in the central nervous system following irradiation with 23-mev X-rays from the betatron. Radiology 62(1):37–46
Kondziolka D, Lunsford LD, Claassen D, Pandalai S, Maitz AH, Flickinger JC (1992) Radiobiology of radiosurgery: part II. The rat C6 glioma model. Neurosurgery 31(2):280–287
Burger PC, Mahley MS Jr, Dudka L, Vogel FS (1979) The morphologic effects of radiation administered therapeutically for intracranial gliomas: a postmortem study of 25 cases. Cancer 44(4):1256–1272
Kureshi SA, Hofman FM, Schneider JH, Chin LS, Apuzzo ML, Hinton DR (1994) Cytokine expression in radiation-induced delayed cerebral injury. Neurosurgery 35(5):822–829
Pizzi M, Sarnico I, Boroni F, Benarese M, Dreano M, Garotta G, Valerio A, Spano P (2004) Prevention of neuron and oligodendrocyte degeneration by interleukin-6 (IL-6) and IL-6 receptor/IL-6 fusion protein in organotypic hippocampal slices. Mol Cell Neurosci 25(2):301–311
Yoshii Y (2008) Pathological review of late cerebral radionecrosis. Brain Tumor Pathol 25(2):51–58
Mizukami Y, Li J, Zhang X, Zimmer MA, Iliopoulos O, Chung DC (2004) Hypoxia-inducible factor-1-independent regulation of vascular endothelial growth factor by hypoxia in colon cancer. Cancer Res 64(5):1765–1772
Pore N, Jiang Z, Gupta A, Cerniglia G, Kao GD, Maity A (2006) EGFR tyrosine kinase inhibitors decrease VEGF expression by both hypoxia-inducible factor (HIF)-1-independent and HIF-1-dependent mechanisms. Cancer Res 66(6):3197–3204
Pore N, Liu S, Shu HK, Li B, Haas-Kogan D, Stokoe D, Milanini-Mongiat J, Pages G, O’Rourke DM, Bernhard E, Maity A (2004) Sp1 is involved in Akt-mediated induction of VEGF expression through an HIF-1-independent mechanism. Mol Biol Cell 15(11):4841–4853
Koch AE, Polverini PJ, Kunkel SL, Harlow LA, DiPietro LA, Elner VM, Elner SG, Strieter RM (1992) Interleukin-8 as a macrophage-derived mediator of angiogenesis. Science 258(5089):1798–1801
Strieter RM, Kunkel SL, Elner VM, Martonyi CL, Koch AE, Polverini PJ, Elner SG (1992) Interleukin-8. A corneal factor that induces neovascularization. Am J Pathol 141(6):1279–1284
Maione TE, Gray GS, Petro J, Hunt AJ, Donner AL, Bauer SI, Carson HF, Sharpe RJ (1990) Inhibition of angiogenesis by recombinant human platelet factor-4 and related peptides. Science 247(4938):77–79
Rempel SA, Dudas S, Ge S, Gutierrez JA (2000) Identification and localization of the cytokine SDF1 and its receptor, CXC chemokine receptor 4, to regions of necrosis and angiogenesis in human glioblastoma. Clin Cancer Res 6(1):102–111
Yang SX, Chen JH, Jiang XF, Wang QL, Chen ZQ, Zhao W, Feng YH, Xin R, Shi JQ, Bian XW (2005) Activation of chemokine receptor CXCR4 in malignant glioma cells promotes the production of vascular endothelial growth factor. Biochem Biophys Res Commun 335(2):523–528
Ping YF, Yao XH, Jiang JY, Zhao LT, Yu SC, Jiang T, Lin MC, Chen JH, Wang B, Zhang R, Cui YH, Qian C, Wang J, Bian XW (2011) The chemokine CXCL12 and its receptor CXCR4 promote glioma stem cell-mediated VEGF production and tumour angiogenesis via PI3K/AKT signalling. J Pathol 224(3):344–354
Li X, Kumar A, Zhang F, Lee C, Li Y, Tang Z, Arjuna P (2010) VEGF-independent angiogenic pathways induced by PDGF-C. Oncotarget 1(4):309–314
Wang D, Huang HJ, Kazlauskas A, Cavenee WK (1999) Induction of vascular endothelial growth factor expression in endothelial cells by platelet-derived growth factor through the activation of phosphatidylinositol 3-kinase. Cancer Res 59(7):1464–1472
Heldin CH, Westermark B (1999) Mechanism of action and in vivo role of platelet-derived growth factor. Physiol Rev 79(4):1283–1316
Miyata T, Toho T, Nonoguchi N, Furuse M, Kuwabara H, Yoritsune E, Kawabata S, Kuroiwa T, Miyatake S (2014) The roles of platelet-derived growth factors and their receptors in brain radiation necrosis. Radiat Oncol 9:51
Reigstad LJ, Varhaug JE, Lillehaug JR (2005) Structural and functional specificities of PDGF-C and PDGF-D, the novel members of the platelet-derived growth factors family. FEBS J 272(22):5723–5741
Glass JP, Hwang TL, Leavens ME, Libshitz HI (1984) Cerebral radiation necrosis following treatment of extracranial malignancies. Cancer 54(9):1966–1972
Glantz MJ, Burger PC, Friedman AH, Radtke RA, Massey EW, Schold SC Jr (1994) Treatment of radiation-induced nervous system injury with heparin and warfarin. Neurology 44(11):2020–2027
Bui QC, Lieber M, Withers HR, Corson K, van Rijnsoever M, Elsaleh H (2004) The efficacy of hyperbaric oxygen therapy in the treatment of radiation-induced late side effects. Int J Radiat Oncol Biol Phys 60(3):871–878
Ohguri T, Imada H, Kohshi K, Kakeda S, Ohnari N, Morioka T, Nakano K, Konda N, Korogi Y (2007) Effect of prophylactic hyperbaric oxygen treatment for radiation-induced brain injury after stereotactic radiosurgery of brain metastases. Int J Radiat Oncol Biol Phys 67(1):248–255
McPherson CM, Warnick RE (2004) Results of contemporary surgical management of radiation necrosis using frameless stereotaxis and intraoperative magnetic resonance imaging. J Neurooncol 68(1):41–47
Gonzalez J, Kumar AJ, Conrad CA, Levin VA (2007) Effect of bevacizumab on radiation necrosis of the brain. Int J Radiat Oncol Biol Phys 67(2):323–326
Torcuator R, Zuniga R, Mohan YS, Rock J, Doyle T, Anderson J, Gutierrez J, Ryu S, Jain R, Rosenblum M, Mikkelsen T (2009) Initial experience with bevacizumab treatment for biopsy confirmed cerebral radiation necrosis. J Neurooncol 94(1):63–68
Wong ET, Huberman M, Lu XQ, Mahadevan A (2008) Bevacizumab reverses cerebral radiation necrosis. J Clin Oncol 26(34):5649–5650
Liu AK, Macy ME, Foreman NK (2009) Bevacizumab as therapy for radiation necrosis in four children with pontine gliomas. Int J Radiat Oncol Biol Phys 75(4):1148–1154
Jeyaretna DS, Curry WT Jr, Batchelor TT, Stemmer-Rachamimov A, Plotkin SR (2011) Exacerbation of cerebral radiation necrosis by bevacizumab. J Clin Oncol 29(7):e159–162
Matuschek C, Bolke E, Nawatny J, Hoffmann TK, Peiper M, Orth K, Gerber PA, Rusnak E, Lammering G, Budach W (2011) Bevacizumab as a treatment option for radiation-induced cerebral necrosis. Strahlenther und Onkol 187(2):135–139
Benoit A, Ducray F, Cartalat-Carel S, Psimaras D, Ricard D, Honnorat J (2011) Favorable outcome with bevacizumab after poor outcome with steroids in a patient with temporal lobe and brainstem radiation necrosis. J Neurol 258(2):328–329
Levin VA, Bidaut L, Hou P, Kumar AJ, Wefel JS, Bekele BN, Grewal J, Prabhu S, Loghin M, Gilbert MR, Jackson EF (2011) Randomized double-blind placebo-controlled trial of bevacizumab therapy for radiation necrosis of the central nervous system. Int J Radiat Oncol Biol Phys 79(5):1487–1495
Acknowledgments
This work was partly supported by a Grant-in-Aid for Scientific Research (C) (26462222) given to M.F. from the Japanese Ministry of Education, Culture, Sports, Science and Technology.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Furuse, M., Nonoguchi, N., Kawabata, S. et al. Delayed brain radiation necrosis: pathological review and new molecular targets for treatment. Med Mol Morphol 48, 183–190 (2015). https://doi.org/10.1007/s00795-015-0123-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00795-015-0123-2