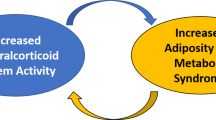
Abstract
Preclinical studies have convincingly demonstrated a role for the mineralocorticoid receptor (MR) in adipose tissue physiology. These studies show that increased MR activation causes adipocyte dysfunction leading to decreased production of insulin-sensitizing products and increased production of inflammatory factors, creating an environment conducive to metabolic and cardiovascular disease. Accumulating data also suggest that MR activation may be an important link between obesity and metabolic syndrome. Moreover, MR activation may mediate the pathogenic consequences of metabolic syndrome. Recent attempts at reversing cardiometabolic damage in patients with type 2 diabetes using MR antagonists have shown promising results. MR antagonists are already used to treat heart failure where their use decreases mortality and morbidity over and above the use of traditional therapies alone. However, more data are needed to establish the benefits of MR antagonists in diabetes, obesity, and metabolic syndrome.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Liu G, Zheng XX, Xu YL, Lu J, Hui RT, Huang XH. Effect of aldosterone antagonists on blood pressure in patients with resistant hypertension: a meta-analysis. J Hum Hypertens. 2015;29(3):159–66.
Dahal K, Kunwar S, Rijal J, Alqatahni F, Panta R, Ishak N et al. The effects of aldosterone antagonists in patients with resistant hypertension: a meta-analysis of randomized and nonrandomized studies. Am J Hypertens. 2015. doi:10.1093/ajh/hpv031
Caprio M, Feve B, Claes A, Viengchareun S, Lombes M, Zennaro MC. Pivotal role of the mineralocorticoid receptor in corticosteroid-induced adipogenesis. FASEB J. 2007;21(9):2185–94.
Caprio M, Antelmi A, Chetrite G, Muscat A, Mammi C, Marzolla V, et al. Antiadipogenic effects of the mineralocorticoid receptor antagonist drospirenone: potential implications for the treatment of metabolic syndrome. Endocrinology. 2011;152(1):113–25.
Guo C, Ricchiuti V, Lian BQ, Yao TM, Coutinho P, Romero JR, et al. Mineralocorticoid receptor blockade reverses obesity-related changes in expression of adiponectin, peroxisome proliferator-activated receptor-gamma, and proinflammatory adipokines. Circulation. 2008;117(17):2253–61.
Li P, Zhang XN, Pan CM, Sun F, Zhu DL, Song HD, et al. Aldosterone perturbs adiponectin and PAI-1 expression and secretion in 3T3-L1 adipocytes. Horm Metab Res. 2011;43(7):464–9.
Hirata A, Maeda N, Hiuge A, Hibuse T, Fujita K, Okada T, et al. Blockade of mineralocorticoid receptor reverses adipocyte dysfunction and insulin resistance in obese mice. Cardiovasc Res. 2009;84(1):164–72.
Armani A, Cinti F, Marzolla V, Morgan J, Cranston GA, Antelmi A, et al. Mineralocorticoid receptor antagonism induces browning of white adipose tissue through impairment of autophagy and prevents adipocyte dysfunction in high-fat-diet-fed mice. FASEB J. 2014;28(8):3745–57. In this study, MR antagonists spironolactone and drospirenone induced up-regulation of brown adipocyte-specific transcripts and increased protein levels of uncoupling protein 1 (UCP1) in visceral and inguinal fat depots of high fat fed mice. Positron emission tomography and magnetic resonance spectroscopy confirmed browning of white adipose tissue. These changes were associated with curbing of high fat diet-induced impairment in glucose tolerance, and with reductions in body weight gain and white fat expansion. Thus, adipocyte MR regulates brown remodeling of white adipose tissue and MR antagonists may prevent the adverse metabolic consequences of adipocyte dysfunction.
Wada T, Kenmochi H, Miyashita Y, Sasaki M, Ojima M, Sasahara M, et al. Spironolactone improves glucose and lipid metabolism by ameliorating hepatic steatosis and inflammation and suppressing enhanced gluconeogenesis induced by high-fat and high-fructose diet. Endocrinology. 2010;151(5):2040–9.
Luo P, Dematteo A, Wang Z, Zhu L, Wang A, Kim HS, et al. Aldosterone deficiency prevents high-fat-feeding-induced hyperglycaemia and adipocyte dysfunction in mice. Diabetologia. 2013;56(4):901–10.
Kuhn E, Bourgeois C, Keo V, Viengchareun S, Muscat A, Meduri G, et al. Paradoxical resistance to high-fat diet-induced obesity and altered macrophage polarization in mineralocorticoid receptor-overexpressing mice. Am J Physiol Endocrinol Metab. 2014;306(1):E75–90.
Garg R, Adler GK. Role of mineralocorticoid receptor in insulin resistance. Curr Opin Endocrinol Diabetes Obes. 2012;19(3):168–75.
Underwood PC, Adler GK. The renin angiotensin aldosterone system and insulin resistance in humans. Curr Hypertens Rep. 2013;15(1):59–70.
Luther JM, Luo P, Kreger MT, Brissova M, Dai C, Whitfield TT, et al. Aldosterone decreases glucose-stimulated insulin secretion in vivo in mice and in murine islets. Diabetologia. 2011;54(8):2152–63.
Bender SB, DeMarco VG, Padilla J, Jenkins NT, Habibi J, Garro M et al. Mineralocorticoid receptor antagonism treats obesity-associated cardiac diastolic dysfunction. Hypertension. 2015;65(5):1082–88. This article demonstrates that treatment of Zucker obese rats with spironolactone improved echocardiographic measures of diastolic dysfunction, reduced cardiac fibrosis, and restored endothelium-dependent vasodilation of isolated coronary arterioles via a nitric oxide-independent mechanism. There was no effect of spironolactone on blood pressure, serum potassium, systemic insulin sensitivity, or proteinuria. These data suggest that MR antagonism can reverse obesity-related cardiac diastolic dysfunction via mechanisms that are independent of blood pressure. These findings are specifically relevant for patients with obesity and insulin resistance.
Schafer N, Lohmann C, Winnik S, van Tits LJ, Miranda MX, Vergopoulos A, et al. Endothelial mineralocorticoid receptor activation mediates endothelial dysfunction in diet-induced obesity. Eur Heart J. 2013;34(45):3515–24. This study showed that endothelial-specific MR gene deletion prevented endothelial dysfunction in two animals animal models of high aldosterone: 1) obese mice (high 'endogenous' aldosterone) and 2) lean mice infused with aldosterone (high’exogenous' aldosterone). Thus, obesity-induced endothelial dysfunction depends on the 'endothelial' MR. MR antagonists may be of therapeutic use in obese patients to decrease vascular dysfunction and subsequent atherosclerotic complications.
Yoshida S, Ishizawa K, Ayuzawa N, Ueda K, Takeuchi M, Kawarazaki W, et al. Local mineralocorticoid receptor activation and the role of Rac1 in obesity-related diabetic kidney disease. Nephron Exp Nephrol. 2014;126(1):16–24. High-glucose stimulation increased Rac1 activity and MR transcriptional activity in cultured mesangial cells from a mouse model of obesity-related type 2 diabetes (KKA(y)). Glucose-induced MR activation was suppressed by over expression of dominant negative Rac1 or the Rac inhibitor EHT1864. In KKA(y) mice, renal Rac1 was activated, and nuclear MR was increased. EHT1864 treatment suppressed renal Rac1 and MR activity and mitigated renal pathology of KKA(y) without changing plasma aldosterone concentration. These results suggest that glucose-induced Rac1 activation, in addition to hyperaldosteronemia, contributes to renal MR activation in diabetes. Along with MR blockade, Rac inhibition may potentially be a preferred option in the treatment of nephropathy in obesity-related diabetic patients.
Tokuyama H, Wakino S, Hara Y, Washida N, Fujimura K, Hosoya K, et al. Role of mineralocorticoid receptor/Rho/Rho-kinase pathway in obesity-related renal injury. Int J Obes (Lond). 2012;36(8):1062–71.
Shibata S, Nagase M, Yoshida S, Kawarazaki W, Kurihara H, Tanaka H, et al. Modification of mineralocorticoid receptor function by Rac1 GTPase: implication in proteinuric kidney disease. Nat Med. 2008;14(12):1370–6.
Huang LL, Nikolic-Paterson DJ, Han Y, Ozols E, Ma FY, Young MJ, et al. Myeloid mineralocorticoid receptor activation contributes to progressive kidney disease. J Am Soc Nephrol. 2014;25(10):2231–40.
Baudrand R, Pojoga LH, Romero JR, Williams GH. Aldosterone's mechanism of action: roles of lysine-specific demethylase 1, caveolin and striatin. Curr Opin Nephrol Hypertens. 2014;23(1):32–7.
Chapman K, Holmes M, Seckl J. 11beta-hydroxysteroid dehydrogenases: intracellular gate-keepers of tissue glucocorticoid action. Physiol Rev. 2013;93(3):1139–206.
Stomby A, Andrew R, Walker BR, Olsson T. Tissue-specific dysregulation of cortisol regeneration by 11betaHSD1 in obesity: has it promised too much? Diabetologia. 2014;57(6):1100–10.
Wang Y, Liu L, Du H, Nagaoka Y, Fan W, Lutfy K, et al. Transgenic overexpression of hexose-6-phosphate dehydrogenase in adipose tissue causes local glucocorticoid amplification and lipolysis in male mice. Am J Physiol Endocrinol Metab. 2014;306(5):E543–51.
Ehrhart-Bornstein M, Lamounier-Zepter V, Schraven A, Langenbach J, Willenberg HS, Barthel A, et al. Human adipocytes secrete mineralocorticoid-releasing factors. Proc Natl Acad Sci U S A. 2003;100(24):14211–6.
Briones AM, Nguyen Dinh Cat A, Callera GE, Yogi A, Burger D, He Y, et al. Adipocytes produce aldosterone through calcineurin-dependent signaling pathways: implications in diabetes mellitus-associated obesity and vascular dysfunction. Hypertension. 2012;59(5):1069–78.
Rios FJ, Neves KB, Nguyen Dinh Cat A, Even S, Palacios R, Montezano AC, et al. Cholesteryl ester-transfer protein inhibitors stimulate aldosterone biosynthesis in adipocytes through Nox-dependent processes. J Pharmacol Exp Ther. 2015;353(1):27–34.
Barter PJ, Caulfield M, Eriksson M, Grundy SM, Kastelein JJ, Komajda M, et al. Effects of torcetrapib in patients at high risk for coronary events. N Engl J Med. 2007;357(21):2109–22.
Grossmann C, Gekle M. Interaction between mineralocorticoid receptor and epidermal growth factor receptor signaling. Mol Cell Endocrinol. 2012;350(2):235–41.
Gomez-Sanchez E, Gomez-Sanchez CE. The multifaceted mineralocorticoid receptor. Compr Physiol. 2014;4(3):965–94.
Rautureau Y, Paradis P, Schiffrin EL. Cross-talk between aldosterone and angiotensin signaling in vascular smooth muscle cells. Steroids. 2011;76(9):834–9.
Barrett Mueller K, Lu Q, Mohammad NN, Luu V, McCurley A, Williams GH, et al. Estrogen receptor inhibits mineralocorticoid receptor transcriptional regulatory function. Endocrinology. 2014;155(11):4461–72. This study showed that estrogen-activated ER inhibits MR-mediated gene transcription from the mouse mammary tumor virus reporter in human embryonic kidney-293 cells. Estradiol also inhibited aldosterone induced intercellular adhesion molecule-1 (ICAM-1) gene transcription thus inhibiting vascular inflammation that contributes atherosclerosis. In contrast, aldosterone activated MR did not affect ER-mediated gene transcription. These data may help to explain the lower incidence of cardiovascular disease in premenopausal women.
Rogerson FM, Yao YZ, Young MJ, Fuller PJ. Identification and characterization of a ligand-selective mineralocorticoid receptor coactivator. FASEB J. 2014;28(10):4200–10.
Young MJ, Rickard AJ. Mineralocorticoid receptors in the heart: lessons from cell-selective transgenic animals. J Endocrinol. 2015;224(1):R1–13.
Usher MG, Duan SZ, Ivaschenko CY, Frieler RA, Berger S, Schutz G, et al. Myeloid mineralocorticoid receptor controls macrophage polarization and cardiovascular hypertrophy and remodeling in mice. J Clin Invest. 2010;120(9):3350–64.
Bienvenu LA, Morgan J, Rickard AJ, Tesch GH, Cranston GA, Fletcher EK, et al. Macrophage mineralocorticoid receptor signaling plays a key role in aldosterone-independent cardiac fibrosis. Endocrinology. 2012;153(7):3416–25.
Li C, Zhang YY, Frieler RA, Zheng XJ, Zhang WC, Sun XN, et al. Myeloid mineralocorticoid receptor deficiency inhibits aortic constriction-induced cardiac hypertrophy in mice. PLoS One. 2014;9(10), e110950.
Weber KT. Aldosterone in congestive heart failure. N Engl J Med. 2001;345(23):1689–97.
Baudrand R, Lian CG, Lian BQ, Ricchiuti V, Yao TM, Li J, et al. Long-term dietary sodium restriction increases adiponectin expression and ameliorates the proinflammatory adipokine profile in obesity. Nutr Metab Cardiovasc Dis. 2014;24(1):34–41.
Bentley-Lewis R, Adler GK, Perlstein T, Seely EW, Hopkins PN, Williams GH, et al. Body mass index predicts aldosterone production in normotensive adults on a high-salt diet. J Clin Endocrinol Metab. 2007;92(11):4472–5.
Sun M, Huang X, Yan Y, Chen J, Wang Z, Xie M, et al. Rac1 is a possible link between obesity and oxidative stress in Chinese overweight adolescents. Obesity (Silver Spring). 2012;20(11):2233–40.
Flores L, Vidal J, Nunez I, Rueda S, Viaplana J, Esmatjes E. Longitudinal changes of blood pressure after weight loss: factors involved. Surg Obes Relat Dis. 2015;11(1):215–21.
Goodfriend TL, Kelley DE, Goodpaster BH, Winters SJ. Visceral obesity and insulin resistance are associated with plasma aldosterone levels in women. Obes Res. 1999;7(4):355–62.
Lo J, Looby SE, Wei J, Adler GK, Grinspoon SK. Increased aldosterone among HIV-infected women with visceral fat accumulation. AIDS. 2009;23(17):2366–70.
Srinivasa S, Fitch KV, Wong K, Torriani M, Mayhew C, Stanley TL et al. Increased aldosterone concentrations are associated with visceral adiposity and insulin resistance among HIV-infected patients. Mineralocorticoids: receptors, hypertension and novel mechanisms. Endocrine Society’s 97th Annual Meeting and Expo, March 5–8, 2015. San Diego, CA, OR38-4.
Hannemann A, Meisinger C, Bidlingmaier M, Doring A, Thorand B, Heier M, et al. Association of plasma aldosterone with the metabolic syndrome in two German populations. Eur J Endocrinol. 2011;164(5):751–8.
Musani SK, Vasan RS, Bidulescu A, Liu J, Xanthakis V, Sims M, et al. Aldosterone, C-reactive protein, and plasma B-type natriuretic peptide are associated with the development of metabolic syndrome and longitudinal changes in metabolic syndrome components: findings from the Jackson Heart Study. Diabetes Care. 2013;36(10):3084–92.
Buglioni A, Cannone V, Cataliotti A, Sangaralingham SJ, Heublein DM, Scott CG, et al. Circulating aldosterone and natriuretic peptides in the general community: relationship to cardiorenal and metabolic disease. Hypertension. 2015;65(1):45–53. In a population based cross sectional study, aldosterone levels in the highest tertile predicted lower natriuretic peptide levels and increased mortality. These data suggest that high aldosterone and lower natriuretic peptides, even within the normal range, may be biomarkers of cardiorenal and metabolic disease in the general community.
Vaidya A, Underwood PC, Hopkins PN, Jeunemaitre X, Ferri C, Williams GH, et al. Abnormal aldosterone physiology and cardiometabolic risk factors. Hypertension. 2013;61(4):886–93.
Brown JM, Underwood PC, Ferri C, Hopkins PN, Williams GH, Adler GK, et al. Aldosterone dysregulation with aging predicts renal vascular function and cardiovascular risk. Hypertension. 2014;63(6):1205–11.
Garg R, Hurwitz S, Williams GH, Hopkins PN, Adler GK. Aldosterone production and insulin resistance in healthy adults. J Clin Endocrinol Metab. 2010;95(4):1986–90.
Catena C, Lapenna R, Baroselli S, Nadalini E, Colussi G, Novello M, et al. Insulin sensitivity in patients with primary aldosteronism: a follow-up study. J Clin Endocrinol Metab. 2006;91(9):3457–63.
Cooper JN, Fried L, Tepper P, Barinas-Mitchell E, Conroy MB, Evans RW, et al. Changes in serum aldosterone are associated with changes in obesity-related factors in normotensive overweight and obese young adults. Hypertens Res. 2013;36(10):895–901.
Garg R, Kneen L, Williams GH, Adler GK. Effect of mineralocorticoid receptor antagonist on insulin resistance and endothelial function in obese subjects. Diabetes Obes Metab. 2014;16(3):268–72.
Hwang MH, Yoo JK, Luttrell M, Kim HK, Meade TH, English M, et al. Mineralocorticoid receptors modulate vascular endothelial function in human obesity. Clin Sci (Lond). 2013;125(11):513–20.
Rotenstein L, Sheridan M, Garg R, Adler G. Effect of mineralocorticoid receptor blockade on hippocampal-dependent memory in obese adults. Obesity (Silver Spring). 2015. doi:10.1002/oby.21104.
Dorey R, Pierard C, Shinkaruk S, Tronche C, Chauveau F, Baudonnat M, et al. Membrane mineralocorticoid but not glucocorticoid receptors of the dorsal hippocampus mediate the rapid effects of corticosterone on memory retrieval. Neuropsychopharmacology. 2011;36(13):2639–49.
Rao AD, Shah RV, Garg R, Abbasi SA, Neilan TG, Perlstein TS, et al. Aldosterone and myocardial extracellular matrix expansion in type 2 diabetes mellitus. Am J Cardiol. 2013;112(1):73–8.
Schjoedt KJ, Rossing K, Juhl TR, Boomsma F, Tarnow L, Rossing P, et al. Beneficial impact of spironolactone on nephrotic range albuminuria in diabetic nephropathy. Kidney Int. 2006;70(3):536–42.
Sato A, Fukuda S. Effect of aldosterone breakthrough on albuminuria during treatment with a direct renin inhibitor and combined effect with a mineralocorticoid receptor antagonist. Hypertens Res. 2013;36(10):879–84.
Mehdi UF, Adams-Huet B, Raskin P, Vega GL, Toto RD. Addition of angiotensin receptor blockade or mineralocorticoid antagonism to maximal angiotensin-converting enzyme inhibition in diabetic nephropathy. J Am Soc Nephrol. 2009;20(12):2641–50.
Oxlund CS, Henriksen JE, Tarnow L, Schousboe K, Gram J, Jacobsen IA. Low dose spironolactone reduces blood pressure in patients with resistant hypertension and type 2 diabetes mellitus: a double blind randomized clinical trial. J Hypertens. 2013;31(10):2094–102.
Garg R, Rao AD, Baimas-George M, Hurwitz S, Foster C, Shah RV, et al. Mineralocorticoid receptor blockade improves coronary microvascular function in individuals with type 2 diabetes. Diabetes. 2015;64(1):236–42. This study showed that 6-month treatment with spironolactone over and above standard therapy with ACEI improves coronary microvascular function in humans with type 2 diabetes as compared to treatment with hydrochlorothiazide or placebo. Coronary flow reserve (CFR), measured by cardiac positron emission tomography was used an indicator of coronary microvascular dysfunction in this study. Since impaired CFR is a predictor of cardiovascular mortality in diabetic patients, MR blockade could have beneficial effects in preventing cardiovascular disease in patients with T2DM.
Murthy VL, Naya M, Foster CR, Gaber M, Hainer J, Klein J, et al. Association between coronary vascular dysfunction and cardiac mortality in patients with and without diabetes mellitus. Circulation. 2012;126(15):1858–68.
Fallo F, Veglio F, Bertello C, Sonino N, Della Mea P, Ermani M, et al. Prevalence and characteristics of the metabolic syndrome in primary aldosteronism. J Clin Endocrinol Metab. 2006;91(2):454–9.
Savard S, Amar L, Plouin PF, Steichen O. Cardiovascular complications associated with primary aldosteronism: a controlled cross-sectional study. Hypertension. 2013;62(2):331–6.
Rossi GP, Cesari M, Cuspidi C, Maiolino G, Cicala MV, Bisogni V, et al. Long-term control of arterial hypertension and regression of left ventricular hypertrophy with treatment of primary aldosteronism. Hypertension. 2013;62(1):62–9.
Chen W, Li F, He C, Zhu Y, Tan W. Elevated prevalence of abnormal glucose metabolism in patients with primary aldosteronism: a meta-analysis. Ir J Med Sci. 2014;183(2):283–91. A meta-analysis including 16 studies showed that the prevalence of elevated glucose in primary aldosteronism was as high as 22.41 %, and this was higher than the prevalence in essential hypertension (OR = 1.55, 95 % CI 1.01-2.36, p = 0.04). Thus, awareness and treatment of pre-diabetic or diabetic states are necessary in caring for individuals with primary aldosteronism.
Catena C, Colussi G, Nadalini E, Chiuch A, Baroselli S, Lapenna R, et al. Cardiovascular outcomes in patients with primary aldosteronism after treatment. Arch Intern Med. 2008;168(1):80–5.
Pitt B, Pfeffer MA, Assmann SF, Boineau R, Anand IS, Claggett B, et al. Spironolactone for heart failure with preserved ejection fraction. N Engl J Med. 2014;370(15):1383–92.
Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med. 1999;341(10):709–17.
Pitt B, White H, Nicolau J, Martinez F, Gheorghiade M, Aschermann M, et al. Eplerenone reduces mortality 30 days after randomization following acute myocardial infarction in patients with left ventricular systolic dysfunction and heart failure. J Am Coll Cardiol. 2005;46(3):425–31.
Krum H, Shi H, Pitt B, McMurray J, Swedberg K, van Veldhuisen DJ, et al. Clinical benefit of eplerenone in patients with mild symptoms of systolic heart failure already receiving optimal best practice background drug therapy: analysis of the EMPHASIS-HF study. Circ Heart Fail. 2013;6(4):711–8.
Vizzardi E, Nodari S, Caretta G, D'Aloia A, Pezzali N, Faden G, et al. Effects of spironolactone on long-term mortality and morbidity in patients with heart failure and mild or no symptoms. Am J Med Sci. 2014;347(4):271–6.
Edelmann F, Wachter R, Schmidt AG, Kraigher-Krainer E, Colantonio C, Kamke W, et al. Effect of spironolactone on diastolic function and exercise capacity in patients with heart failure with preserved ejection fraction: the Aldo-DHF randomized controlled trial. JAMA. 2013;309(8):781–91.
Ferreira JP, Santos M, Almeida S, Marques I, Bettencourt P, Carvalho H. Mineralocorticoid receptor antagonism in acutely decompensated chronic heart failure. Eur J Intern Med. 2014;25(1):67–72.
Pfeffer MA, Claggett B, Assmann SF, Boineau R, Anand IS, Clausell N, et al. Regional variation in patients and outcomes in the Treatment of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist (TOPCAT) trial. Circulation. 2015;131(1):34–42.
Rossignol P, Dobre D, McMurray JJ, Swedberg K, Krum H, van Veldhuisen DJ, et al. Incidence, determinants, and prognostic significance of hyperkalemia and worsening renal function in patients with heart failure receiving the mineralocorticoid receptor antagonist eplerenone or placebo in addition to optimal medical therapy: results from the Eplerenone in Mild Patients Hospitalization and Survival Study in Heart Failure (EMPHASIS-HF). Circ Heart Fail. 2014;7(1):51–8.
Vardeny O, Wu DH, Desai A, Rossignol P, Zannad F, Pitt B, et al. Influence of baseline and worsening renal function on efficacy of spironolactone in patients with severe heart failure: insights from RALES (Randomized Aldactone Evaluation Study). J Am Coll Cardiol. 2012;60(20):2082–9.
Rossignol P, Cleland JG, Bhandari S, Tala S, Gustafsson F, Fay R, et al. Determinants and consequences of renal function variations with aldosterone blocker therapy in heart failure patients after myocardial infarction: insights from the Eplerenone Post-Acute Myocardial Infarction Heart Failure Efficacy and Survival Study. Circulation. 2012;125(2):271–9.
Eschalier R, McMurray JJ, Swedberg K, van Veldhuisen DJ, Krum H, Pocock SJ, et al. Safety and efficacy of eplerenone in patients at high risk for hyperkalemia and/or worsening renal function: analyses of the EMPHASIS-HF study subgroups (Eplerenone in Mild Patients Hospitalization And Survival Study in Heart Failure). J Am Coll Cardiol. 2013;62(17):1585–93.
Matsumoto Y, Mori Y, Kageyama S, Arihara K, Sugiyama T, Ohmura H, et al. Spironolactone reduces cardiovascular and cerebrovascular morbidity and mortality in hemodialysis patients. J Am Coll Cardiol. 2014;63(6):528–36. This study enrolled 309 oligoanuric hemodialysis patients to assess whether spironolactone treatment reduces the incidence of cardiovascular and cerebrovascular morbidity and mortality. After 3-year follow-up, the primary outcome (composite of death or hospitalization for cardiovascular or cerebrovascular events) occurred in 5.7% of patients in the spironolactone (25 mg daily) group and in 12.5% of patients in the control group (p = 0.016). Serious hyperkalemia led to treatment discontinuation in only 3 patients (1.9%). Thus MR blockade using spironolactone may substantially reduce the risk of cardiovascular and cerebrovascular disease among hemodialysis patients.
Acknowledgments
This work was supported in part by NIH grant K24 HL103845
Compliance with Ethics Guidelines
ᅟ
Conflict of Interest
Gail K. Adler declares personal fees from Pfizer, Japan. Rajesh Garg declares no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Topical Collection on Hypertension and the Kidney
Rights and permissions
About this article
Cite this article
Garg, R., Adler, G.K. Aldosterone and the Mineralocorticoid Receptor: Risk Factors for Cardiometabolic Disorders. Curr Hypertens Rep 17, 52 (2015). https://doi.org/10.1007/s11906-015-0567-8
Published:
DOI: https://doi.org/10.1007/s11906-015-0567-8