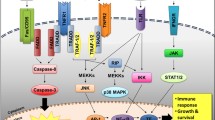
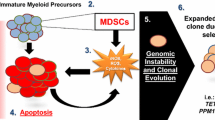
Abstract
Purpose of Review
Immune dysregulation is a defining feature of myelodysplastic syndromes (MDS). Recently, several studies have further defined the complex role of immune alterations within MDS. Herein, we will summarize some of these findings and discuss the therapeutic strategies currently in development.
Recent Findings
Immune alterations in MDS are complex, heterogeneous, and intertwined with clonal hematopoiesis and stromal cell dysfunction. Inflammation in MDS proceeds as a vicious cycle, mediated in large part by secreted factors, which induce cell death and activate innate immune signaling. Therapeutic targeting of this variable immune dysregulation has led to modest responses thus far, but incorporation of the growing repertoire of immunotherapy brings new potential for improved outcomes.
Summary
The immune milieu is variable across the spectrum of MDS subtypes, with a changing balance of inflammatory and suppressive cellular forces from low- to high-risk disease.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major Importance
Arber DA, Orazi A, Hasserjian R, Thiele J, Borowitz MJ, Le Beau MM, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127(20):2391–405. https://doi.org/10.1182/blood-2016-03-643544.
• Kittang AO, Kordasti S, Sand KE, Costantini B, Kramer AM, Perezabellan P, et al. Expansion of myeloid derived suppressor cells correlates with number of T regulatory cells and disease progression in myelodysplastic syndrome. Oncoimmunology. 2016;5(2):e1062208. https://doi.org/10.1080/2162402x.2015.1062208. Expansion of MDSCs stimulates production of Tregs and occurs concurrently with disease progression.
Kordasti SY, Ingram W, Hayden J, Darling D, Barber L, Afzali B, et al. CD4+CD25high Foxp3+ regulatory T cells in myelodysplastic syndrome (MDS). Blood. 2007;110(3):847–50. https://doi.org/10.1182/blood-2007-01-067546.
Ganan-Gomez I, Wei Y, Starczynowski DT, Colla S, Yang H, Cabrero-Calvo M, et al. Deregulation of innate immune and inflammatory signaling in myelodysplastic syndromes. Leukemia. 2015;29(7):1458–69. https://doi.org/10.1038/leu.2015.69.
Li AJ, Calvi LM. The microenvironment in myelodysplastic syndromes: niche-mediated disease initiation and progression. Exp Hematol. 2017;55:3–18. https://doi.org/10.1016/j.exphem.2017.08.003.
Epling-Burnette PK, Bai F, Painter JS, Rollison DE, Salih HR, Krusch M, et al. Reduced natural killer (NK) function associated with high-risk myelodysplastic syndrome (MDS) and reduced expression of activating NK receptors. Blood. 2007;109(11):4816–24. https://doi.org/10.1182/blood-2006-07-035519.
Shetty V, Mundle S, Alvi S, Showel M, Broady-Robinson L, Dar S, et al. Measurement of apoptosis, proliferation and three cytokines in 46 patients with myelodysplastic syndromes. Leuk Res. 1996;20(11–12):891–900.
Mundle SD, Venugopal P, Cartlidge JD, Pandav DV, Broady-Robinson L, Gezer S, et al. Indication of an involvement of interleukin-1 beta converting enzyme-like protease in intramedullary apoptotic cell death in the bone marrow of patients with myelodysplastic syndromes. Blood. 1996;88(7):2640–7.
Seipelt G, Ganser A, Duranceyk H, Maurer A, Ottmann OG, Hoelzer D. Induction of TNF-alpha in patients with myelodysplastic syndromes undergoing treatment with interleukin-3. Br J Haematol. 1993;84(4):749–51.
Alexandrakis M, Coulocheri S, Xylouri I, Ganotakis E, Eliakis P, Karkavitsas N, et al. Elevated serum TNF-alpha concentrations are predictive of shortened survival in patients with high-risk myelodysplastic syndromes. Haematologia (Budap). 1998;29(1):13–24.
Sawanobori M, Yamaguchi S, Hasegawa M, Inoue M, Suzuki K, Kamiyama R, et al. Expression of TNF receptors and related signaling molecules in the bone marrow from patients with myelodysplastic syndromes. Leuk Res. 2003;27(7):583–91.
Stifter G, Heiss S, Gastl G, Tzankov A, Stauder R. Over-expression of tumor necrosis factor-alpha in bone marrow biopsies from patients with myelodysplastic syndromes: relationship to anemia and prognosis. Eur J Haematol. 2005;75(6):485–91. https://doi.org/10.1111/j.1600-0609.2005.00551.x.
• Cluzeau T, McGraw KL, Irvine B, Masala E, Ades L, Basiorka AA, et al. Pro-inflammatory proteins S100A9 and TNFalpha suppress erythropoietin elaboration in myelodysplastic syndromes. Haematologica. 2017; https://doi.org/10.3324/haematol.2016.158857. High levels of S100A9 and TNFα suppress transcription and cellular elaboration of EPO.
Claessens YE, Bouscary D, Dupont JM, Picard F, Melle J, Gisselbrecht S, et al. In vitro proliferation and differentiation of erythroid progenitors from patients with myelodysplastic syndromes: evidence for Fas-dependent apoptosis. Blood. 2002;99(5):1594–601.
Ribeiro E, Lima CS, Metze K, Lorand-Metze I. Flow cytometric analysis of the expression of Fas/Fasl in bone marrow CD34+ cells in myelodysplastic syndromes: relation to disease progression. Leuk Lymphoma. 2004;45(2):309–13.
Gersuk GM, Beckham C, Loken MR, Kiener P, Anderson JE, Farrand A, et al. A role for tumour necrosis factor-alpha, Fas and Fas-Ligand in marrow failure associated with myelodysplastic syndrome. Br J Haematol. 1998;103(1):176–88.
Bouscary D, De Vos J, Guesnu M, Jondeau K, Viguier F, Melle J, et al. Fas/Apo-1 (CD95) expression and apoptosis in patients with myelodysplastic syndromes. Leukemia. 1997;11(6):839–45.
Gupta P, Niehans GA, LeRoy SC, Gupta K, Morrison VA, Schultz C, et al. Fas ligand expression in the bone marrow in myelodysplastic syndromes correlates with FAB subtype and anemia, and predicts survival. Leukemia. 1999;13(1):44–53.
Gyan E, Frisan E, Beyne-Rauzy O, Deschemin JC, Pierre-Eugene C, Randriamampita C, et al. Spontaneous and Fas-induced apoptosis of low-grade MDS erythroid precursors involves the endoplasmic reticulum. Leukemia. 2008;22(10):1864–73. https://doi.org/10.1038/leu.2008.172.
Garcia-Manero G, Gartenberg G, Steensma DP, Schipperus MR, Breems DA, de Paz R, et al. A phase 2, randomized, double-blind, multicenter study comparing siltuximab plus best supportive care (BSC) with placebo plus BSC in anemic patients with International Prognostic Scoring System low- or intermediate-1-risk myelodysplastic syndrome. Am J Hematol. 2014;89(9):E156–62. https://doi.org/10.1002/ajh.23780.
Nemeth E, Rivera S, Gabayan V, Keller C, Taudorf S, Pedersen BK, et al. IL-6 mediates hypoferremia of inflammation by inducing the synthesis of the iron regulatory hormone hepcidin. J Clin Invest. 2004;113(9):1271–6. https://doi.org/10.1172/JCI20945.
Schipperus MR, Sonneveld P, Lindemans J, van Lom K, Vlastuin M, Abels J. Interleukin-6 and interleukin-1 enhancement of GM-CSF-dependent proliferation of haematopoietic progenitor cells in myelodysplastic syndromes. Br J Haematol. 1991;77(4):515–22.
Tsimberidou AM, Estey E, Wen S, Pierce S, Kantarjian H, Albitar M, et al. The prognostic significance of cytokine levels in newly diagnosed acute myeloid leukemia and high-risk myelodysplastic syndromes. Cancer. 2008;113(7):1605–13. https://doi.org/10.1002/cncr.23785.
Meyers CA, Albitar M, Estey E. Cognitive impairment, fatigue, and cytokine levels in patients with acute myelogenous leukemia or myelodysplastic syndrome. Cancer. 2005;104(4):788–93. https://doi.org/10.1002/cncr.21234.
• Basiorka AA, McGraw KL, Eksioglu EA, Chen X, Johnson J, Zhang L, et al. The NLRP3 inflammasome functions as a driver of the myelodysplastic syndrome phenotype. Blood. 2016;128(25):2960–75. https://doi.org/10.1182/blood-2016-07-730556. NLRP3 inflammasome drives clonal expansion and pryoptotic cell death via S100A9.
Sallman DA, Cluzeau T, Basiorka AA, List A. Unraveling the pathogenesis of MDS: the NLRP3 inflammasome and pyroptosis drive the MDS phenotype. Front Oncol. 2016;6:151. https://doi.org/10.3389/fonc.2016.00151.
• Schneider RK, Schenone M, Ferreira MV, Kramann R, Joyce CE, Hartigan C, et al. Rps14 haploinsufficiency causes a block in erythroid differentiation mediated by S100A8 and S100A9. Nat Med. 2016;22(3):288–97. https://doi.org/10.1038/nm.4047. Connects genetic alteration with S100A8/9 and immune dysregulation.
• Mei Y, Zhao B, Basiorka AA, Yang J, Cao L, Zhang J, et al. Age-related inflammatory bone marrow microenvironment induces ineffective erythropoiesis mimicking del(5q) MDS. Leukemia. 2017; https://doi.org/10.1038/leu.2017.326. mDia1 and miR-146a knock out mimics 5q deletion syndrome with increased TNFα and IL-6 secretion and MDSC expansion.
Cull AH, Snetsinger B, Buckstein R, Wells RA, Rauh MJ. Tet2 restrains inflammatory gene expression in macrophages. Exp Hematol. 2017;55:56–70 e13. https://doi.org/10.1016/j.exphem.2017.08.001.
•• Jaiswal S, Natarajan P, Silver AJ, Gibson CJ, Bick AG, Shvartz E, et al. Clonal hematopoiesis and risk of atherosclerotic cardiovascular disease. N Engl J Med. 2017;377(2):111–21. https://doi.org/10.1056/NEJMoa1701719. Clonal hematopoiesis leads to twofold higher risk of early onset myocardial infarction.
Cull AH, Mahendru D, Snetsinger B, Good D, Tyryshkin K, Chesney A, et al. Overexpression of Arginase 1 is linked to DNMT3A and TET2 mutations in lower-grade myelodysplastic syndromes and chronic myelomonocytic leukemia. Leuk Res. 2017;65:5–13. https://doi.org/10.1016/j.leukres.2017.12.003.
Pellagatti A, Esoof N, Watkins F, Langford CF, Vetrie D, Campbell LJ, et al. Gene expression profiling in the myelodysplastic syndromes using cDNA microarray technology. Br J Haematol. 2004;125(5):576–83. https://doi.org/10.1111/j.1365-2141.2004.04958.x.
Maratheftis CI, Andreakos E, Moutsopoulos HM, Voulgarelis M. Toll-like receptor-4 is up-regulated in hematopoietic progenitor cells and contributes to increased apoptosis in myelodysplastic syndromes. Clin Cancer Res. 2007;13(4):1154–60. https://doi.org/10.1158/1078-0432.ccr-06-2108.
Wei Y, Dimicoli S, Bueso-Ramos C, Chen R, Yang H, Neuberg D, et al. Toll-like receptor alterations in myelodysplastic syndrome. Leukemia. 2013;27(9):1832–40. https://doi.org/10.1038/leu.2013.180.
Velegraki M, Papakonstanti E, Mavroudi I, Psyllaki M, Tsatsanis C, Oulas A, et al. Impaired clearance of apoptotic cells leads to HMGB1 release in the bone marrow of patients with myelodysplastic syndromes and induces TLR4-mediated cytokine production. Haematologica. 2013;98(8):1206–15. https://doi.org/10.3324/haematol.2012.064642.
Varney ME, Melgar K, Niederkorn M, Smith M, Barreyro L, Starczynowski DT. Deconstructing innate immune signaling in myelodysplastic syndromes. Exp Hematol. 2015;43(8):587–98. https://doi.org/10.1016/j.exphem.2015.05.016.
Chen X, Eksioglu EA, Zhou J, Zhang L, Djeu J, Fortenbery N, et al. Induction of myelodysplasia by myeloid-derived suppressor cells. J Clin Invest. 2013;123(11):4595–611. https://doi.org/10.1172/JCI67580.
• Fang J, Bolanos LC, Choi K, Liu X, Christie S, Akunuru S, et al. Ubiquitination of hnRNPA1 by TRAF6 links chronic innate immune signaling with myelodysplasia. Nat Immunol. 2017;18(2):236–45. https://doi.org/10.1038/ni.3654. Overactivation of TLR pathway via TRAF6 signaling leads to splicing defects and subsequently impaired hematopoiesis.
Breccia M, Alimena G. NF-kappaB as a potential therapeutic target in myelodysplastic syndromes and acute myeloid leukemia. Expert Opin Ther Targets. 2010;14(11):1157–76. https://doi.org/10.1517/14728222.2010.522570.
Culver-Cochran AE, Starczynowski DT. Chronic innate immune signaling results in ubiquitination of splicing machinery. Cell Cycle. 2018;17:1–4. https://doi.org/10.1080/15384101.2018.1429082.
Rhyasen GW, Bolanos L, Fang J, Jerez A, Wunderlich M, Rigolino C, et al. Targeting IRAK1 as a therapeutic approach for myelodysplastic syndrome. Cancer Cell. 2013;24(1):90–104. https://doi.org/10.1016/j.ccr.2013.05.006.
Li J, Volk A, Zhang J, Cannova J, Dai S, Hao C, et al. Sensitizing leukemia stem cells to NF-kappaB inhibitor treatment in vivo by inactivation of both TNF and IL-1 signaling. Oncotarget. 2017;8(5):8420–35. https://doi.org/10.18632/oncotarget.14220.
• Carey A, Edwards DK, Eide CA, Newell L, Traer E, Medeiros BC, et al. Identification of Interleukin-1 by functional screening as a key mediator of cellular expansion and disease progression in acute myeloid leukemia. Cell Rep. 2017;18(13):3204–18. https://doi.org/10.1016/j.celrep.2017.03.018. IL-1 is identified in a functional screen as a driver of progression in high risk MDS and AML and p38 inhibition reversed IL-1-induced progression and growth.
Kordasti SY, Afzali B, Lim Z, Ingram W, Hayden J, Barber L, et al. IL-17-producing CD4(+) T cells, pro-inflammatory cytokines and apoptosis are increased in low risk myelodysplastic syndrome. Br J Haematol. 2009;145(1):64–72. https://doi.org/10.1111/j.1365-2141.2009.07593.x.
Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9(3):162–74. https://doi.org/10.1038/nri2506.
Perazzio AS, Oliveira JS, Figueiredo VL, Chauffaille ML. Increase of IRF-1 gene expression and impairment of T regulatory cells suppression activity on patients with myelodysplastic syndrome: a longitudinal one-year study. Leuk Res. 2017;55:6–17. https://doi.org/10.1016/j.leukres.2017.01.008.
Aggarwal N, Swerdlow SH, TenEyck SP, Boyiadzis M, Felgar RE. Natural killer cell (NK) subsets and NK-like T-cell populations in acute myeloid leukemias and myelodysplastic syndromes. Cytometry B Clin Cytom. 2016;90(4):349–57. https://doi.org/10.1002/cyto.b.21349.
Marcondes AM, Mhyre AJ, Stirewalt DL, Kim SH, Dinarello CA, Deeg HJ. Dysregulation of IL-32 in myelodysplastic syndrome and chronic myelomonocytic leukemia modulates apoptosis and impairs NK function. Proc Natl Acad Sci U S A. 2008;105(8):2865–70. https://doi.org/10.1073/pnas.0712391105.
Szczepanski MJ, Szajnik M, Welsh A, Whiteside TL, Boyiadzis M. Blast-derived microvesicles in sera from patients with acute myeloid leukemia suppress natural killer cell function via membrane-associated transforming growth factor-beta1. Haematologica. 2011;96(9):1302–9. https://doi.org/10.3324/haematol.2010.039743.
• Medyouf H, Mossner M, Jann JC, Nolte F, Raffel S, Herrmann C, et al. Myelodysplastic cells in patients reprogram mesenchymal stromal cells to establish a transplantable stem cell niche disease unit. Cell Stem Cell. 2014;14(6):824–37. https://doi.org/10.1016/j.stem.2014.02.014. Stromal cells are both altered in MDS and critical components of the bone marrow niche that sustain mutant clones.
Raaijmakers MH. Disease progression in myelodysplastic syndromes: do mesenchymal cells pave the way? Cell Stem Cell. 2014;14(6):695–7. https://doi.org/10.1016/j.stem.2014.05.010.
Bhagat TD, Chen S, Bartenstein M, Barlowe AT, Von Ahrens D, Choudhary GS, et al. Epigenetically aberrant stroma in MDS propagates disease via Wnt/beta-catenin activation. Cancer Res. 2017;77(18):4846–57. https://doi.org/10.1158/0008-5472.can-17-0282.
• Chen S, Zambetti NA, Bindels EM, Kenswill K, Mylona AM, Adisty NM, et al. Massive parallel RNA sequencing of highly purified mesenchymal elements in low-risk MDS reveals tissue-context-dependent activation of inflammatory programs. Leukemia. 2016;30(9):1938–42. https://doi.org/10.1038/leu.2016.91. RNA sequencing reveals specfic inflammatory signatures within stromal cells from MDS patients.
Raaijmakers MH, Mukherjee S, Guo S, Zhang S, Kobayashi T, Schoonmaker JA, et al. Bone progenitor dysfunction induces myelodysplasia and secondary leukaemia. Nature. 2010;464(7290):852–7. https://doi.org/10.1038/nature08851.
Lim ZY, Killick S, Germing U, Cavenagh J, Culligan D, Bacigalupo A, et al. Low IPSS score and bone marrow hypocellularity in MDS patients predict hematological responses to antithymocyte globulin. Leukemia. 2007;21(7):1436–41. https://doi.org/10.1038/sj.leu.2404747.
Haidinger M, Geyeregger R, Poglitsch M, Weichhart T, Zeyda M, Vodenik B, et al. Antithymocyte globulin impairs T-cell/antigen-presenting cell interaction: disruption of immunological synapse and conjugate formation. Transplantation. 2007;84(1):117–21. https://doi.org/10.1097/01.tp.0000266677.45428.80.
Matsuda S, Koyasu S. Mechanisms of action of cyclosporine. Immunopharmacology. 2000;47(2–3):119–25.
Sloand EM, Rezvani K. The role of the immune system in myelodysplasia: implications for therapy. Semin Hematol. 2008;45(1):39–48. https://doi.org/10.1053/j.seminhematol.2007.11.006.
Parikh AR, Olnes MJ, Barrett AJ. Immunomodulatory treatment of myelodysplastic syndromes: antithymocyte globulin, cyclosporine, and alemtuzumab. Semin Hematol. 2012;49(4):304–11. https://doi.org/10.1053/j.seminhematol.2012.07.004.
Haider M, Al Ali N, Padron E, Epling-Burnette P, Lancet J, List A, et al. Immunosuppressive therapy: exploring an underutilized treatment option for myelodysplastic syndrome. Clin Lymphoma Myeloma Leuk. 2016;16(16 Suppl):S44–8. https://doi.org/10.1016/j.clml.2016.02.017.
Stahl M, Deveaux M, de Witte TMM, Neukirchen J, Sekeres MA, Brunner AM, et al. The use of immunosuppressive therapy (IST) in patients with the myelodysplastic syndromes (MDS): clinical outcomes and their predictors in a large international patient cohort. Blood. 2017;130(Suppl 1):422.
Deeg HJ, Gotlib J, Beckham C, Dugan K, Holmberg L, Schubert M, et al. Soluble TNF receptor fusion protein (etanercept) for the treatment of myelodysplastic syndrome: a pilot study. Leukemia. 2002;16(2):162–4. https://doi.org/10.1038/sj.leu.2402356.
Baron F, Suciu S, Amadori S, Muus P, Zwierzina H, Denzlinger C, et al. Value of infliximab (Remicade(R)) in patients with low-risk myelodysplastic syndrome: final results of a randomized phase II trial (EORTC trial 06023) of the EORTC Leukemia Group. Haematologica. 2012;97(4):529–33. https://doi.org/10.3324/haematol.2011.044347.
Scott BL, Ramakrishnan A, Storer B, Becker PS, Petersdorf S, Estey EH, et al. Prolonged responses in patients with MDS and CMML treated with azacitidine and etanercept. Br J Haematol. 2010;148(6):944–7. https://doi.org/10.1111/j.1365-2141.2009.08061.x.
Scott BL, Ramakrishnan A, Fosdal M, Storer B, Becker P, Petersdorf S, et al. Anti-thymocyte globulin plus etanercept as therapy for myelodysplastic syndromes (MDS): a phase II study. Br J Haematol. 2010;149(5):706–10. https://doi.org/10.1111/j.1365-2141.2010.08145.x.
Mies A, Hermine O, Platzbecker U. Activin receptor II ligand traps and their therapeutic potential in myelodysplastic syndromes with ring sideroblasts. Curr Hematol Malig Rep. 2016;11(6):416–24. https://doi.org/10.1007/s11899-016-0347-9.
Platzbecker U, Germing U, Gotze KS, Kiewe P, Mayer K, Chromik J, et al. Luspatercept for the treatment of anaemia in patients with lower-risk myelodysplastic syndromes (PACE-MDS): a multicentre, open-label phase 2 dose-finding study with long-term extension study. Lancet Oncol. 2017;18(10):1338–47. https://doi.org/10.1016/S1470-2045(17)30615-0.
Schinke C, Giricz O, Li W, Shastri A, Gordon S, Barreyro L, et al. IL8-CXCR2 pathway inhibition as a therapeutic strategy against MDS and AML stem cells. Blood. 2015;125(20):3144–52. https://doi.org/10.1182/blood-2015-01-621631.
Navas TA, Mohindru M, Estes M, Ma JY, Sokol L, Pahanish P, et al. Inhibition of overactivated p38 MAPK can restore hematopoiesis in myelodysplastic syndrome progenitors. Blood. 2006;108(13):4170–7. https://doi.org/10.1182/blood-2006-05-023093.
Shastri A, Will B, Steidl U, Verma A. Stem and progenitor cell alterations in myelodysplastic syndromes. Blood. 2017;129(12):1586–94. https://doi.org/10.1182/blood-2016-10-696062.
Navas T, Zhou L, Estes M, Haghnazari E, Nguyen AN, Mo Y, et al. Inhibition of p38alpha MAPK disrupts the pathological loop of proinflammatory factor production in the myelodysplastic syndrome bone marrow microenvironment. Leuk Lymphoma. 2008;49(10):1963–75. https://doi.org/10.1080/10428190802322919.
Sokol L, Cripe L, Kantarjian H, Sekeres MA, Parmar S, Greenberg P, et al. Randomized, dose-escalation study of the p38α MAPK inhibitor SCIO-469 in patients with myelodysplastic syndrome. Leukemia. 2013;27(4):977–80. https://doi.org/10.1038/leu.2012.264.
• Bachegowda L, Morrone K, Winski SL, Mantzaris I, Bartenstein M, Ramachandra N, et al. Pexmetinib: a novel dual inhibitor of Tie2 and p38 MAPK with efficacy in preclinical models of myelodysplastic syndromes and acute myeloid leukemia. Cancer Res. 2016;76(16):4841–9. https://doi.org/10.1158/0008-5472.CAN-15-3062. Overexpression of p38 is found in all MDS sub-types and p38 inhibition with pexmetinib is effective in vitro.
Garcia-Manero G, Khoury HJ, Jabbour E, Lancet J, Winski SL, Cable L, et al. A phase I study of oral ARRY-614, a p38 MAPK/Tie2 dual inhibitor, in patients with low or intermediate-1 risk myelodysplastic syndromes. Clin Cancer Res. 2015;21(5):985–94. https://doi.org/10.1158/1078-0432.CCR-14-1765.
Reilly M, Miller RM, Thomson MH, Patris V, Ryle P, McLoughlin L, et al. Randomized, double-blind, placebo-controlled, dose-escalating phase I, healthy subjects study of intravenous OPN-305, a humanized anti-TLR2 antibody. Clin Pharmacol Ther. 2013;94(5):593–600. https://doi.org/10.1038/clpt.2013.150.
Park JS, Gamboni-Robertson F, He Q, Svetkauskaite D, Kim JY, Strassheim D, et al. High mobility group box 1 protein interacts with multiple Toll-like receptors. Am J Physiol Cell Physiol. 2006;290(3):C917–24. https://doi.org/10.1152/ajpcell.00401.2005.
Lee KL, Ambler CM, Anderson DR, Boscoe BP, Bree AG, Brodfuehrer JI, et al. Discovery of clinical candidate 1-{[(2S,3S,4S)-3-ethyl-4-fluoro-5-oxopyrrolidin-2-yl]methoxy}-7-methoxyisoquinoline-6-carboxamide (PF-06650833), a potent, selective inhibitor of interleukin-1 receptor associated kinase 4 (IRAK4), by fragment-based drug design. J Med Chem. 2017;60(13):5521–42. https://doi.org/10.1021/acs.jmedchem.7b00231.
Eksioglu EA, Chen X, Heider KH, Rueter B, McGraw KL, Basiorka AA, et al. Novel therapeutic approach to improve hematopoiesis in low risk MDS by targeting MDSCs with the Fc-engineered CD33 antibody BI 836858. Leukemia. 2017;31(10):2172–80. https://doi.org/10.1038/leu.2017.21.
Brayer J, Lancet JE, Powers J, List A, Balducci L, Komrokji R, et al. WT1 vaccination in AML and MDS: a pilot trial with synthetic analog peptides. Am J Hematol. 2015;90(7):602–7. https://doi.org/10.1002/ajh.24014.
Keilholz U, Letsch A, Busse A, Asemissen AM, Bauer S, Blau IW, et al. A clinical and immunologic phase 2 trial of Wilms tumor gene product 1 (WT1) peptide vaccination in patients with AML and MDS. Blood. 2009;113(26):6541–8. https://doi.org/10.1182/blood-2009-02-202598.
Griffiths EA, Srivastava P, Matsuzaki J, Brumberger Z, Wang ES, Kocent J, et al. NY-ESO-1 vaccination in combination with decitabine induces antigen-specific T-lymphocyte responses in patients with myelodysplastic syndrome. Clin Cancer Res. 2017;24:1019–29. https://doi.org/10.1158/1078-0432.CCR-17-1792.
Rezvani K, Yong AS, Mielke S, Savani BN, Musse L, Superata J, et al. Leukemia-associated antigen-specific T-cell responses following combined PR1 and WT1 peptide vaccination in patients with myeloid malignancies. Blood. 2008;111(1):236–42. https://doi.org/10.1182/blood-2007-08-108241.
Qazilbash MH, Wieder E, Thall PF, Wang X, Rios R, Lu S, et al. PR1 peptide vaccine induces specific immunity with clinical responses in myeloid malignancies. Leukemia. 2017;31(3):697–704. https://doi.org/10.1038/leu.2016.254.
Rezvani K, Yong AS, Mielke S, Jafarpour B, Savani BN, Le RQ, et al. Repeated PR1 and WT1 peptide vaccination in Montanide-adjuvant fails to induce sustained high-avidity, epitope-specific CD8+ T cells in myeloid malignancies. Haematologica. 2011;96(3):432–40. https://doi.org/10.3324/haematol.2010.031674.
Choi DC, Tremblay D, Iancu-Rubin C, Mascarenhas J. Programmed cell death-1 pathway inhibition in myeloid malignancies: implications for myeloproliferative neoplasms. Ann Hematol. 2017;96(6):919–27. https://doi.org/10.1007/s00277-016-2915-4.
Boddu P, Kantarjian H, Garcia-Manero G, Allison J, Sharma P, Daver N. The emerging role of immune checkpoint based approaches in AML and MDS. Leuk Lymphoma. 2017;59:1–13. https://doi.org/10.1080/10428194.2017.1344905.
Kondo A, Yamashita T, Tamura H, Zhao W, Tsuji T, Shimizu M, et al. Interferon-gamma and tumor necrosis factor-alpha induce an immunoinhibitory molecule, B7-H1, via nuclear factor-kappaB activation in blasts in myelodysplastic syndromes. Blood. 2010;116(7):1124–31. https://doi.org/10.1182/blood-2009-12-255125.
• Yang H, Bueso-Ramos C, DiNardo C, Estecio MR, Davanlou M, Geng QR, et al. Expression of PD-L1, PD-L2, PD-1 and CTLA4 in myelodysplastic syndromes is enhanced by treatment with hypomethylating agents. Leukemia. 2014;28(6):1280–8. https://doi.org/10.1038/leu.2013.355. Checkpoint receptors are increased following HMA and offer a new sub-group that may benefit from checkpoint inhibition.
Garcia-Manero G, Tallman MS, Martinelli G, Ribrag V, Yang H, Balakumaran A, et al. Pembrolizumab, a PD-1 inhibitor, in patients with myelodysplastic syndrome (MDS) after failure of hypomethylating agent treatment. Blood. 2016;128(22):345.
Green C, Yan M, Nalle S, Ma C, Robert A, Zhong J, et al. Evidence of targetable immune dysfunction in the bone marrow of patients with intermediate/high-risk myelodysplastic syndrome refractory to hypomethylating agents. Blood. 2017;130(Suppl 1):4241.
Stahl M, Gedrich R, Peck R, LaVallee T, Eder JP. Targeting KIT on innate immune cells to enhance the antitumor activity of checkpoint inhibitors. Immunotherapy. 2016;8(7):767–74. https://doi.org/10.2217/imt-2016-0040.
• Gleason MK, Ross JA, Warlick ED, Lund TC, Verneris MR, Wiernik A, et al. CD16xCD33 bispecific killer cell engager (BiKE) activates NK cells against primary MDS and MDSC CD33+ targets. Blood. 2014;123(19):3016–26. https://doi.org/10.1182/blood-2013-10-533398. Study showing BiKE therapy is effective in vitro.
•• Vallera DA, Felices M, McElmurry R, McCullar V, Zhou X, Schmohl JU, et al. IL15 trispecific killer engagers (TriKE) make natural killer cells specific to CD33+ targets while also inducing persistence, in vivo expansion, and enhanced function. Clin Cancer Res. 2016;22(14):3440–50. https://doi.org/10.1158/1078-0432.CCR-15-2710. TriKEs are superior to BiKEs in vitro in that they restore potent NK cell function.
Batlevi CL, Matsuki E, Brentjens RJ, Younes A. Novel immunotherapies in lymphoid malignancies. Nat Rev Clin Oncol. 2016;13(1):25–40. https://doi.org/10.1038/nrclinonc.2015.187.
Rettig MP, Godwin J, Vey N, Fox B, Ballesteros-Merino C, Bifulco CB, et al. Preliminary translational results from an ongoing phase 1 study of flotetuzumab, a CD123 x CD3 Dart®, in AML/MDS: rationale for combining flotetuzumab and anti-PD-1/PD-L1 immunotherapies. Blood. 2017;130(Suppl 1):1365.
Dolstra H, Roeven MWH, Spanholtz J, Hangalapura BN, Tordoir M, Maas F, et al. Successful transfer of umbilical cord blood CD34+ hematopoietic stem and progenitor-derived NK cells in older acute myeloid leukemia patients. Clin Cancer Res. 2017;23(15):4107–18. https://doi.org/10.1158/1078-0432.CCR-16-2981.
• Bjorklund AT, Carlsten M, Sohlberg E, Liu LL, Clancy T, Karimi M, et al. Complete remission with reduction of high-risk clones following haploidentical NK cell therapy against MDS and AML. Clin Cancer Res. 2018; https://doi.org/10.1158/1078-0432.Ccr-17-3196. First clinical trial to show efficacy of NK cells in high-risk MDS.
Sarvaria A, Jawdat D, Madrigal JA, Saudemont A. Umbilical cord blood natural killer cells, their characteristics, and potential clinical applications. Front Immunol. 2017;8:329. https://doi.org/10.3389/fimmu.2017.00329.
Domogala A, Blundell M, Thrasher A, Lowdell MW, Madrigal JA, Saudemont A. Natural killer cells differentiated in vitro from cord blood CD34+ cells are more advantageous for use as an immunotherapy than peripheral blood and cord blood natural killer cells. Cytotherapy. 2017;19(6):710–20. https://doi.org/10.1016/j.jcyt.2017.03.068.
Lieberman NAP, DeGolier K, Haberthur K, Chinn H, Moyes KW, Bouchlaka MN, et al. An uncoupling of canonical phenotypic markers and functional potency of ex vivo-expanded natural killer cells. Front Immunol. 2018;9:150. https://doi.org/10.3389/fimmu.2018.00150.
Wagner JA, Rosario M, Romee R, Berrien-Elliott MM, Schneider SE, Leong JW, et al. CD56bright NK cells exhibit potent antitumor responses following IL-15 priming. J Clin Invest. 2017;127(11):4042–58. https://doi.org/10.1172/JCI90387.
Zhang W, Stevens BM, Budde EE, Forman SJ, Jordan CT, Purev E. Anti-CD123 CAR T-cell therapy for the treatment of myelodysplastic syndrome. Blood. 2017;130(Suppl 1):1917.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Kathryn S. Ivy and P. Brent Ferrell, Jr. declare that they have no relevant conflicts of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Myelodysplastic Syndromes
Rights and permissions
About this article
Cite this article
Ivy, K.S., Brent Ferrell, P. Disordered Immune Regulation and its Therapeutic Targeting in Myelodysplastic Syndromes. Curr Hematol Malig Rep 13, 244–255 (2018). https://doi.org/10.1007/s11899-018-0463-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11899-018-0463-9