Abstract
Purpose of Review
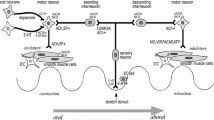
Chronic constipation is a common, nonmotor, and prodromal symptom in Parkinson’s disease (PD). Its underlying neuropathology may provide pathophysiological insight into PD. Here, we critically review what is currently known about the neuroanatomical and brain-gut interactions, and the origin and progression of Lewy pathology (LP) at three levels—brain/brainstem, spinal cord, and enteric nervous system.
Recent Findings
Many recent studies have illustrated the challenges of examining LP in tissues obtained from colon biopsies of PD patients. Large-scale epidemiological studies have not confirmed the widely accepted Braakpostula.
Summary
In this review, we propose an alternative origin and route of spread of LP in PD. We describe novel, noninvasive neurophysiological testing that could advance the understanding of LP and complex bidirectional brain-pelvic floor neural pathways in PD—a true disease model of a neurogastrointestinal disorder. This review may provide the impetus for future studies investigating gut and brain interaction and constipation in PD.



Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: ••Of major importance
Parkinson J. An essay on the shaking palsy. Neely and Jones, London: Sherwood; 1817.
Kaye J, Gage H, Kimbler A, et al. Escess burden of constipation in Parkinson’s disease: a pilot study. Mov Disord. 2006;21(8):1270–3. https://doi.org/10.1002/mds.20942.
Siddiqui MF, Rast S, Lynn MJ, et al. Autonomic dysfunction in Parkinson’s disease: a comprehensive symptom survey. Parkinsonism Relat Disor. 2002;8:2777–84.
Postuma RB, Gagnon JF, Pelletier A, Montplaisir J. Prodromal autonomic symptoms and signs of Parkinson’s disease and dementia with Lewy bodies. Mov Disord. 2013;28(5):597–604. https://doi.org/10.1002/mds.25445.
Lin C, Lin J, Liu Y, et al. Risk of Parkinson’s disease following severe constipation: a nationwide population-based cohort study. Parkinsonism Relat Disord. 2014;20(12):1371–5. https://doi.org/10.1016/j.parkreldis.2014.09.026.
Jost WH. Gastrointestinal dysfunction in Parkinson’s disease. J Neurol Sci. 2010;289(1-2):69–73. https://doi.org/10.1016/j.jns.2009.08.020.
Gage H, Kaye J, Kimber A, Storey L, Egan M, Qiao Y, et al. Correlates of constipation in people with Parkinson’s. Parkinsonism Relat Disord. 2011;17(2):106–11. https://doi.org/10.1016/j.parkreldis.2010.11.003.
Edwards LL, Quigley EM, Harned RK, Hofman R, Pfeiffer RF. Characterization of swallowing and defecation in Parkinson’s disease. Am J Gastroenterol. 1994;89(1):15–25.
Hall, John E (2011). “General principles of gastrointestinal function” Guyton and Hal Textbook of Medical Physiology (12th Ed.) Saunders Elsevier. p 755.
Gerson MD. The enteric nervous system: a second brain. Hospital Practice (1995). 1999;34(7):31–2. 35–8,41–2
Glazer EJ, Basbaum AI. Leucine encephalin: localization in and axoplasmic transport by sacral parasympathetic neurons. Science. 1980;208(4451):1479–81. https://doi.org/10.1126/science.6155697.
Kawatani M, Shioda S, Nakai Y, Takeshige C, de Groat WC. Ultrastructural analysis of enkephalinergic terminals in parasympathetic ganglia innervating the urinary bladder of the cat. J Comp Neurol. 1989;288(1):81–91. https://doi.org/10.1002/cne.902880107.
Holstege G, Tan J. Supraspinal control of motorneurons innervating the striated muscles of the pelvic floor including the urethral and anal sphincters in the cat. Brain. 1987;110(5):1323–44. https://doi.org/10.1093/brain/110.5.1323.
Mannen T. Neuropathological findings of Onuf’s nucleus and its significance. Neuropathology. 2000;20(s1):30–3. https://doi.org/10.1046/j.1440-1789.2000.00298.x.
Del Tredici K, Rub U, De Vos RA, et al. Where does Parkinson disease pathology begin in the brain? J Neuropathol Exp Neurol. 2002;61(5):413–26. https://doi.org/10.1093/jnen/61.5.413.
Cersosimo MG, Benarroch EE. Neural control of the gastrointestinal tract: implications for Parkinson disease. Mov Disord. 2008;23(8):1065–73. https://doi.org/10.1002/mds.22051.
Browning KN, Travalgi RA. Central nervous control of the gastrointestinal motility and secretion and modulation of gastrointestinal functions. Compr Physiol. 2014;4(4):1339–68. https://doi.org/10.1002/cphy.c130055.
Janig W, McLachlan EM. Organization of lumbar spinal outflow to distal colon and pelvic organs. Physiol Rev. 1987;67(4):1332–404. https://doi.org/10.1152/physrev.1987.67.4.1332.
Cookson MR, Bandmann O. Parkinson’s disease: insights from pathways. Hum Mol Genet. 2010;19:821–7.
Burre J. Alpha-synuclein promotes SNARE-complex assembly in vivo and in vitro. Science. 2010;329(5999):1663–7. https://doi.org/10.1126/science.1195227.
Braak H, Ghebremedhin E, Rub U, et al. Stages in the development of Parkinson’s disease-related pathology. Cell Tissue Res. 2004;318(1):121–34. https://doi.org/10.1007/s00441-004-0956-9.
Del Tredici K, Braak H. Spinal cord lesions in sporadic Parkinson’s disease. Acta Neurpathol. 2012;124(5):643–64. https://doi.org/10.1007/s00401-012-1028-y.
Hilton D, Stephens M, Kirk L, Edwards P, Potter R, Zajicek J, et al. Accumulation of alpha-synuclein in the bowel of patients in the pre-clinical phase of Parkinson’s disease. Acta Neuropathol. 2014;127(2):235–41. https://doi.org/10.1007/s00401-013-1214-6.
Shannon KM, Keshavarzian A, Dodiya H, et al. Is alpha-synuclein in the colon a biomarker for premotor Parkinson’s disease? Evidence from 3 cases. Mov Disord. 2012;27(6):716–9. https://doi.org/10.1002/mds.25020.
•• Ruffman C, Parkkinen L. Gut feelings about alpha-synuclein in gastrointestinal biopsies: biomarker in the making? Mov Disord. 2016;31(2):193–202. Excellent explanation why readily source of tissue from colonic biopsies not specific enough for PD
Lebouvier T, Chaumette T, Damier P, Coron E, Touchefeu Y, Vrignaud S, et al. Pathological lesions in colonic biopsies during Parkinson’s disease. Gut. 2008;57(12):1741–3. https://doi.org/10.1136/gut.2008.162503.
Lebouvier T, Neunlist M, Bruley d, Varannes S, et al. Colonic biopsies to assess the neuropathology of Parkinson’s disease and its relationship with symptoms. PLoS One. 2010;5(9):e12728. https://doi.org/10.1371/journal.pone.0012728.
Pouclet H, Lebouvier T, Coron E, Bruley des Varannes S, Rouaud T, Roy M, et al. A comparison between rectal and colonic biopsies to detect Lewy pathology in Parkinson’s disease. Neurobiol Dis. 2012;45(1):305–9. https://doi.org/10.1016/j.nbd.2011.08.014.
Gold A, Turkalp ZT, Munoz DG. Enteric alpha-synuclein expression is increased in Parkinson’s disease but not Alzeimer’s disease. Mov Disord. 2013;28(2):237–40. https://doi.org/10.1002/mds.25298.
Visanji NJ, Marras C, Kern DS, et al. Colonic mucosal a-synuclein lacks specificity as biomarker for Parkinson’s disease. Neurology. 2015;84(6):609–16. https://doi.org/10.1212/WNL.0000000000001240.
Braak H, de Vos RA, Boh J, et al. Gastric alpha-synuclein immunoreactive inclusions in Meissner’s and Auerbach’s plexuses in cases staged for Parkinson’s disease-related brain bathology. Neurosci Lett. 2006;396(1):67–72. https://doi.org/10.1016/j.neulet.2005.11.012.
Beach TG, Adler CH, Sue LI, et al. Multi-organ distribution of phosphorylated alpha-synuclein histopathology in subjects with Lewy body disorders. Acta Neuropathol. 2010;119(6):689–702. https://doi.org/10.1007/s00401-010-0664-3.
Gray MT, Munoz DG, Gray DA, Schlossmacher MG, Woulfe JM. Alpha-synuclein in the appendiceal mucosa of neurologically intact subjects. Mov Disord. 2014;29(8):991–8. https://doi.org/10.1002/mds.25779.
Nakai M, Fujita M, Waragai M, Sugama S, Wei J, Akatsu H, et al. Experssion of alpha-synuclein, a presynaptic protein implicated in Parkinson’s disease, in erythropoetic lineage. Biochem Biophys Res Comm. 2007;358(1):104–10. https://doi.org/10.1016/j.bbrc.2007.04.108.
Tamo W, Imaizumi T, Tanji K, Yoshida H, Mori F, Yoshimoto M, et al. Expression of alpha-synuclein, the precursor of non-amyloid ceta component of Alzheimer’s disease amyloid, in human cerebral blood vessels. Neurosci Lett. 2002;326(1):5–8. https://doi.org/10.1016/S0304-3940(02)00297-5.
Askanas V, Engel WK, Alzarez RB, et al. Novel immunolocalization of alpha-synuclein in human muscle of inclusion-body myositis, regenerating and necrotic muscle fibers, and at neuromuscular junctions. J Neuropathol Exp Neurol. 2000;59(7):592–8. https://doi.org/10.1093/jnen/59.7.592.
Paillusson S, Clairembault T, Biraud M, Neunlist M, Derkinderen P. Activity-dependent secretion of alpha-synuclein by enteric neurons. J Neurochem. 2013;125(4):512–7. https://doi.org/10.1111/jnc.12131.
Stolzenberg E, Berry D, Yang D, Lee EY, Kroemer A, Kaufman S, et al. A role for neuronal alpha-synuclein in gastrointestinal immunity. J Innate Immun. 2017;9(5):456–63. https://doi.org/10.1159/000477990.
Wang S, Chu CH, Stewart T, Ginghina C, Wang Y, Nie H, et al. Alpha-synuclein, a chemoattractant, directs microglial activation via H2O2-dependent Lyn phosphorylation. Proc Natl Acad Sci U S A. 2015;112(15):E1926–35. https://doi.org/10.1073/pnas.1417883112.
Lebouvier T, Coron E, Chaumette T, Paillusson S, Bruley des Varannes S, Neunlist M, et al. Routine colonic biopsies as a new tool to study the enteric nervous system in living patients. Neurogastroenterol Motil. 2010;22(1):e11–4. https://doi.org/10.1111/j.1365-2982.2009.01368.x.
Barshop K, Willingham FF, Brugge WR, Zukerberg LR, Kuo B. Endoscopic mucosal resection is superior to rectal suction biopsy for analysis of enteric ganglia in constipation and dysmotility. Gastrointest Endosc. 2017;S0016-5107(17):32246–0. https://doi.org/10.1016/j.gie.2017.08.037.
Braak H, Del Tredici K, Rub U, et al. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging. 2003;24(2):197–211. https://doi.org/10.1016/S0197-4580(02)00065-9.
Braak H, Rub U, Gai WP, et al. Idiopathic Parkinson’s disease: possible routes by which vulnerable neuronal types may be subject to neuroinvasion by an unknown pathogen. J Neural Transm. 2003;110(5):517–36. https://doi.org/10.1007/s00702-002-0808-2.
Svensson E, Horvath-Pubo E, Thomsen RW, et al. Vagotomy and subsequent risk of Parkinson’s disease. Ann Neurol. 2015;78(4):522–9. https://doi.org/10.1002/ana.24448.
Tysnes OB, Kenborg L, Herlofson K, Steding-Jessen M, Horn A, Olsen JH, et al. Does vagotomy reduce the risk of Parkinson’s disease? Ann Neurol. 2015;78(6):1011–2. https://doi.org/10.1002/ana.24531.
•• Liu B, Fang F, Pedersen NL, et al. Vagotomy and Parkinson disease. A Swedish register-based matched-cohort study. Neurology. 2017;88:1996–2002. Latest large-scale epidemiological study showing that vagotomy is not protective against the development of PD overall.
Johnston D. Operative mortality and postoperative morbidity of highly selective vagotomy. Br Med J. 1975;4(5996):545–7. https://doi.org/10.1136/bmj.4.5996.545.
Berg D, Postuma RB, Bloem B, Chan P, Dubois B, Gasser T, et al. Time to redefine PD? Introductory statement of the MDS Task Force on the definition of Parkinson’s disease. Mov Disord. 2014;29(4):454–62. https://doi.org/10.1002/mds.25844.
Xilouri M, Brekk OR, Stefanis L. Autophagy and alpha-synuclein: relevance to Parkinson’s disease and related synucleopathies. Mov Disord. 2016;31(2):178–92. https://doi.org/10.1002/mds.26477.
Aziz Q, Thompson DG. Brain-gut axis in health and disease. Gastroenterology. 1998;114(3):559–78. https://doi.org/10.1016/S0016-5085(98)70540-2.
Hobson AR, Aziz Q. Brain imaging and functional gastrointestinal disorders: has it helped our understanding. Gut. 2004;53(8):1198–206. https://doi.org/10.1136/gut.2003.035642.
•• Remes-Troche JM, Tantiphlachiva K, Attaluri A, et al. A bi-directional assessment of the human brain-anorectal axis. Neurogastroenterol Motil. 2011;23(3):240-e118. Comprehensive description of bidirectional brain-gut neurophysiological studies that could be useful in PD.
Loening-Bauke V, Read NW, Yamada T. Cerebral evoked potentials after rectal stimulation. Electroencephalogr Clin Neurophysiol. 1991;80(6):490–5. https://doi.org/10.1016/0168-5597(91)90130-P.
Hobday DL, Hobson AR, Sarkar S, et al. Cortical processing of human gut sensation: an evoked potential study. Am J Physiol Gastrointest Liver Physiol. 2002;283:335–9.
Drewes AM, Dimcevski G, Sami S, et al. The “human visceral homunculus” to pain evoked in the oesophagus, stomach, duodenum and sigmoid colon. Exp Brain Res. 2006;174(3):443–52. https://doi.org/10.1007/s00221-006-0480-0.
Chan YK, Herkes GK, Badcock C, Evans PR, Bennett E, Kellow JE. Alterations in cerebral potentials evoked by rectal distension in irritable bowel syndrome. Am J Gastroenterol. 2001;96(8):2413–7. https://doi.org/10.1111/j.1572-0241.2001.04088.x.
Sinhamahapatra P, Saha SP, Chowdhury A, Chakrabarti SK, Ghosh A, Maiti B. Visceral afferent hypersensitivity in irritable bowel syndrome-evaluation by cerebral evoked potential after rectal stimulation. Am J Gastroenterol. 2001;96(7):2150–7. https://doi.org/10.1111/j.1572-0241.2001.03952.x.
Paine PA, Aziz Q, Gardener E, Hobson A, Mistry S, Thompson DG, et al. Assessing the temporal reproducibility of human esophageal motor-evoked potentials to transcranial magnetic stimulation. J Clin Neurophysiol. 2006;23(4):374–80. https://doi.org/10.1097/01.wnp.0000209578.08391.e2.
Tantiphlachiva K, Attaluri A, Valestin J, Yamada T, Rao SSC. Translumbar and transsacral motor-evoked potentials: a novel test for spino-anorectal neuropathy in spinal cord injury. Am J Gastroenterol. 2011;106(5):907–14. https://doi.org/10.1038/ajg.2010.478.
Rao SSC. Dyssynergic defecation and biofeedback therapy. Gastroenterol Clin N Am. 2008;37(3):569–86. https://doi.org/10.1016/j.gtc.2008.06.011.
Ashraf W, Wazolek ZK, Pfeiffer RF, et al. Anorectal function in fluctuating (on-off) Parkinson’s disease: evaluation by combined anorectal manometry and electromyography. Mov Disord. 1995;10(5):650–7. https://doi.org/10.1002/mds.870100519.
•• Su A, Gandhy R, Barlow C, et al. Utility of high-resolution anorectal manometry and wireless motility capsule in the evaluation of patients with Parkinson’s disease and chronic constipation. BMJ Open Gastro. 2016;3(1):e000118. Recent GI transit and anorectal manometric evaluation of large cohort of PD patients
Rao SSC, Valesin J, Brown CK, et al. Long-term efficacy of biofeedback therapy for dyssynergic defecation: randomized controlled trial. Am J Gastroenterol. 2010;105(4):890–6. https://doi.org/10.1038/ajg.2010.53.
Tateno F, Sakakibara R, Yokoi Y, Kishi M, Ogawa E, Uchiyama T, et al. Levadopa ameliorated anorectal constipation in de novo Parkinson’s disease: the QL-GAT study. Parkinsonism Relat Disord. 2011;17(9):662–6. https://doi.org/10.1016/j.parkreldis.2011.06.002.
Edwards LL, Quigley EM, Harned RK, et al. Defecatory response to apomorphine. Ann Neurol. 1993;33(5):490–3. https://doi.org/10.1002/ana.410330512.
Albanese A, Brisinda G, Bentivoglio AR, Maria G. Treatment of outlet obstruction constipation in Parkinson’s disease with botulinum neurotoxin A. Am J Gastroenterol. 2003;98(6):1439–40. https://doi.org/10.1111/j.1572-0241.2003.07514.x.
Knudsen K, Fedorova TD, Bekker AC, Iversen P, Østergaard K, Krogh K, et al. Objective colonic dysfunction is far more prevalent than subjective constipation in Parkinson’s disease: a colonic transit and volume study. J Parkinsons Dis. 2017;7(2):359–67. https://doi.org/10.3233/JPD-161050.
Sakakibara R, Odaka T, Uchiyama T, Asahina M, Yamaguchi K, Yamaguchi T, et al. Colonic transit time and rectoanal videomanometry in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2003;74(2):268–72. https://doi.org/10.1136/jnnp.74.2.268.
Rao SS, Camilleri M, Hasler WL, et al. Evaluation of gastrointesinal transit in clinical practice: position paper of the American and European neurogastroenterology and motility societies. Neurogastroenterol Motil. 2011;23(1):8–25. https://doi.org/10.1111/j.1365-2982.2010.01612.x.
Su A, Gandhy R, Barlow C, Triadafilopoulos G. Utility of the wireless motility capsule and lactulose breath testing in the evaluation of patients with Parkinson’s disease who present with functional gastrointestinal symptoms. BMJ Open Gastro. 2017;4(1):e0001323. https://doi.org/10.1136/bmjgast-2017-000132.
Zangaglia R, Maritignoni E, Glorioso M, et al. Macrogol for the treatment of constipation in Parkinson’s disease. A randomized placebo-controlled study. Mov Disord. 2007;22(9):1239–44. https://doi.org/10.1002/mds.21243.
Ondo WG, Kenney C, Sullivan K, Davidson A, Hunter C, Jahan I, et al. Placebo-controlled trial of lubiprostone for constipation associated with Parkinson disease. Neurology. 2012;78(21):1650–4. https://doi.org/10.1212/WNL.0b013e3182574f28.
Barichelia M, Pacchetti C, Bolliri C, et al. Probiotics and prebiotic fiber for constipation associated with Pakinson disease: an RCT. Neurology. 2016;87(12):1274–80. https://doi.org/10.1212/WNL.0000000000003127.
Jost WH, Schimrigk K. The effect of cisapride on delayed colonic transit time in patients with idiopathic Parkinson’s disease. Wien Klin Wochenschr. 1994;106(21):673–6.
Jost WH, Schimrigk K. Long-term results with cisapride in Parkinson’s disease. Mov Disord. 1997;12(3):423–5. https://doi.org/10.1002/mds.870120324.
Morgan JC, Kapil S. Tegaserod in constipation associated with Parkinson’s disease. Clin Neuropharmacol. 2007;30(1):52–4. https://doi.org/10.1097/01.WNF.0000240942.21499.97.
Lui Z, Sakakibara R, Odaka T, et al. Mosapride citrate, a novel 5-HT4 agonist and partial 5-HT3 antagonist, ameliorates constipation in parkinsonian patients. Mov Disord. 2005;20:680–6.
Acknowledgements
We would like to acknowledge research support provided by the Parkinson’s Foundation through the 2017 CCRA Translational Clinical Research Grant. Furthermore, we would like to acknowledge Joe Kelley and the CSRA Parkinson Support Group for their engagement and support of our research.
Financial Disclosures
Amol Sharma serves on the advisory board for Ironwood Pharmaceuticals. JCM has received honoraria for speaking for Acadia, Impax, and Teva. He has served as a consultant for Acadia, Cynapsus, Impax, Neurocrine, Parkinson’s Foundation, and Teva. He has also received compensation for serving as an expert witness in various neurological legal cases. He has served as a site PI or sub-I for clinical trials with Abbvie, Acadia, Acorda, Biotie, CHDI, Cynapsus, Impax, Kyowa, Lilly, Lundbeck, NIH, Parkinson’s Foundation, PSG, Roche, and Serina. SSCR received research support from Ironwood/Forrest Pharmaceuticals and Intone Medical. SSCR serves on the advisory board for Ironwood/Forrest Pharmaceuticals, Synergy Pharmaceuticals, Salix Pharmaceuticals, Orphomed, and Vibrant Ltd.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflicts of interest.
Additional information
This article is part of the Topical Collection on Neurogastroenterology and Motility Disorders of the Gastrointestinal Tract
Rights and permissions
About this article
Cite this article
Sharma, A., Kurek, J., Morgan, J.C. et al. Constipation in Parkinson’s Disease: a Nuisance or Nuanced Answer to the Pathophysiological Puzzle?. Curr Gastroenterol Rep 20, 1 (2018). https://doi.org/10.1007/s11894-018-0609-x
Published:
DOI: https://doi.org/10.1007/s11894-018-0609-x