Abstract
Purpose of review
This article provides an overview of the clinical presentation, investigations, and treatment options for gastrointestinal tract (GIT) dysfunction in patients with Parkinson’s disease (PD) and other movement disorders.
Recent findings
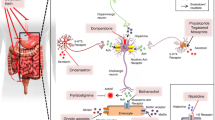
GIT dysfunction commonly appears as constipation and fecal incontinence (mostly overflow, accompanied with sphincter failure in multiple system atrophy [MSA]). Bowel dysfunction (underactive) occurs irrespectively from the site of the neurologic lesion, which is in contrast to site-dependent bladder dysfunction (brain, overactive; periphery, underactive). GI emergencies may arise, including intestinal pseudo-obstruction, intussusception, volvulus, and stercoral ulcer (ulcer of the colon due to pressure and irritation resulting from severe, prolonged constipation). Bowel function tests in neurologic patients often show a combination of slow transit and anorectal dysfunction. Management for slow transit constipation includes bulking agents, softening agents, yogurt/probiotics, and prokinetic agents. Suppositories, botulinum toxin injections, and transanal irrigation are options for managing anorectal constipation.
Conclusions
Function of the bowel is commonly affected in PD and other movement disorders. Neurologists play an important role in assessing bowel symptoms in their patients and planning treatment strategies, often in collaboration with specialist teams.




Similar content being viewed by others
References
Panicker JN, Sakakibara R (2020) Lower urinary tract and bowel dysfunction in neurologic disease. Continuum (Minneap Minn) 26:178–199
Chaudhuri KR, Healy DG, Schapira AHV, National Institute for Clinical Excellence (2006) Non-motor symptoms of Parkinson’s disease: diagnosis and management. Lancet Neurol 5:235–245
Sakakibara R, Uchiyama T, Yamanishi T, Shirai K, Hattori T (2008) Bladder and bowel dysfunction in Parkinson’s disease. J Neural Transm (Vienna) 115:443–460
Costa M, Brookes SJH, Hennig GW (2000) Anatomy and physiology of the enteric nervous system. Gut 47(Suppl IV):iv15–iv19
Hansen MB (2003) Neurohumoral control of gastrointestinal motility. Physiol Res 52:1–30
Beck G, Hori Y, Hayashi Y, Morii E, Takehara T, Mochizuki H (2020) Detection of phosphorylated alpha-synuclein in the muscularis propria of the gastrointestinal tract is a sensitive predictor for Parkinson’s disease. Park Dis 2020:4687530. https://doi.org/10.1155/2020/4687530 eCollection 2020
Ohlsson B, Englund E (2019) Atrophic myenteric and submucosal neurons are observed in Parkinson’s disease. Park Dis 2019:7935820. https://doi.org/10.1155/2019/7935820 eCollection 2019
Sakakibara R, Awa Y, Naya Y, Tobe T, Uchiyama T, Hattori T (2007) Neobladder overactivity; an equivalent to spontaneous rectal contraction. Int J Urol 14:1054–1056
Wakabayashi K, Takahachi H, Ohama E, Ikuta F (1990) Parkinson’s disease: an immunohistochemical study of Lewy body-containing neurons in the enteric nervous system. Acta Neuropathol 79:581–583
Nathan PW, Smith MC (1953) Spinal pathways subserving defaecation and sensation from the lower bowel. J Neurol Neurosurg Psychiatry 16:245–256
Kanesaka T, Sakakibara R, Ito S, Ito T, Odaka T, Yamaguchi T, Uchiyama T, Liu Z, Yamamoto T, Hattori T (2006) Intestinal pseudo-obstruction in acute myelitis. Intern Med 45:35–36
Lynch AC, Frizelle FA (2006) Colorectal motility and defecation after spinal cord injury in humans. Prog Brain Res 152:335–343
Ito T, Sakakibara R, Sakakibara Y, Mori M, Hattori T (2004) Medulla and gut. Intern Med 43:1091
Sawai S, Sakakibara R, Kanai K, Kawaguchi N, Uchiyama T, Yamamoto T, Ito T, Liu Z, Hattori T (2006) Isolated vomiting due to a unilateral dorsal vagal complex lesion. Eur Neurol 56:246–248
Hyun JW, Kwon YN, Kim SM, Lee HL, Jeong WK, Lee HJ, Kim BJ, Kim SW, Shin HY, Shin HJ, Oh SY, Huh SY, Kim W, Park MS, Oh J, Jang H, Park NY, Lee MY, Kim SH, Kim HJ (2020) Value of area postrema syndrome in differentiating adults with AQP4 vs. MOG antibodies. Front Neurol 11:396. https://doi.org/10.3389/fneur.2020.00396
Wakabayashi K, Takahashi H (1997) Neuropathology of autonomic nervous system in Parkinson’s disease. Eur Neurol 38(Suppl 2):2–7
Nakamori H, Naitou K, Horii Y, Shimaoka H, Horii K, Sakai H, Yamada A, Furue H, Shiina T, Shimizu Y (2019) Roles of the noradrenergic nucleus locus coeruleus and dopaminergic nucleus A11 region as supraspinal defecation centers in rats. Am J Physiol Gastrointest Liver Physiol 317:G545–G555
Tateno F, Sakakibara R, Kishi M, Tsuyusaki Y, Furukawa R, Yoshimatsu Y, Suzuki Y (2012) Brainstem stroke and increased anal tone. Lower Urin Tract Symptoms 4:161–163
Bove C, Anselmi L, Travagli RA (2019) Altered gastric tone and motility response to brain-stem dopamine in a rat model of parkinsonism. Am J Physiol Gastrointest Liver Physiol 317:G1–G7
Anselmi L, Toti L, Bove C, Hampton J, Travagli RA (2017) A nigro-vagal pathway controls gastric motility and is affected in a rat model of parkinsonism. Gastroenterology 153:1581–1593
Arai E, Arai M, Uchiyama T, Higuchi Y, Aoyagi K, Yamanaka Y, Yamamoto T, Nagano O, Shiina A, Maruoka D, Matsumura T, Nakagawa T, Katsuno T, Imazeki F, Saeki N, Kuwabara S, Yokosuka O (2012) Subthalamic deep brain stimulation can improve gastric emptying in Parkinson’s disease. Brain 135:1478–1485
Ameer NH, Lee JH, Choi MA, Jin GS, Kim MS, Park BR (2010) The effects of glutamate NMDA receptor antagonist mk-801 on gastrointestinal motility after middle cerebral artery occlusion in rats. Korean J Physiol Pharmacol 14:151–156
Levinthal DJ, Strick PL (2020) Multiple areas of the cerebral cortex influence the stomach. Proc Natl Acad Sci U S A 117:13078–13083
Jones MP, Dilley JB, Drossman D, Crowell MD (2006) Brain-gut connections in functional GI disorders: anatomic and physiologic relationships. Neurogastroenterol Motil 18:91–103
Griffiths DJ, Fowler CJ (2013) The micturition switch and its forebrain influences. Acta Physiol (Oxf) 207:93–109
Shiina S, Sakakibara R, Doi H, Tateno F, Sato M, Masaka T, Kishi M, Tsuyusaki Y, Ogata T, Aiba Y, Tateno H (2015) Levodopa does not worsen gastric emptying in Parkinson’s disease. J Am Geriatr Soc 63:2185–2186
Sakakibara R, Shinotoh H, Uchiyama T, Sakuma M, Kashiwado M, Yoshiyama M, Hattori T (2001) Questionnaire-based assessment of pelvic organ dysfunction in Parkinson’s disease. Auton Neurosci 92:76–85
Doi H, Sakakibara R, Sato M, Masaka T, Kishi M, Tateno A, Tateno F, Tsuyusaki Y, Takahashi O (2012) Plasma levodopa peak delay and impaired gastric emptying in Parkinson’s disease. J Neurol Sci 319:86–88
Tateno F, Sakakibara R, Kishi M, Ogawa E, Yoshimatsu Y, Takada N, Suzuki Y, Mouri T, Uchiyama T, Yamamoto T (2011) Incidence of emergency intestinal pseudo-obstruction in Parkinson’s disease. J Am Geriatr Soc 59:2373–2375
Tateno F, Sakakibara R, Aiba Y, Tsuyusaki Y, Kishi M, Tateno H, Ogata T (2016) Stercoral ulcer and colonic perforation in an individual with Parkinson’s disease with constipation. J Am Geriatr Soc 64:e118–e120
Tateno F, Sakakibara R, Aiba Y, Ogata T, Katsumata M, Matsuoka Y (2020) Recurrent sigmoid volvulus in a patient with Parkinson’s disease. Clin Auton Res 30:283–285
Doi H, Sakakibara R, Masuda M, Tateno F, Aiba Y, Kishi M, Yamanishi T, Yamamoto T, Matsuoka K (2019) Gastrointestinal function in dementia with Lewy bodies: a comparison with Parkinson disease. Clin Auton Res 29:633–638
Sakakibara R, Tateno F, Aiba Y, Ogata T, Kishi M, Terada H, Inaoka T, Nakatsuka T, Matsuoka K (2018) MIBG myocardial scintigraphy identifies premotor PD/DLB during a negative DAT scan period: second report. Mov Disord Clin Pract 6:46–50
Coon EA, Mandrekar JN, Berini SE, Benarroch EE, Sandroni P, Low PA, Singer W (2020) Predicting phenoconversion in pure autonomic failure. Neurology 95(7):e889–e897. https://doi.org/10.1212/WNL.0000000000010002
Sakakibara R, Doi H, Fukudo S (2019) Lewy body constipation. J Anus Rectum Colon 3:10–17
Abbott RD, Petrovitch H, White LR, Masaki KH, Tanner CM, Curb JD et al (2001) Frequency of bowel movements and the future risk of Parkinson’s disease. Neurology 57:456–462
Hawkes CH, Del Tredici K, Braak H (2010) A timeline for Parkinson’s disease. Parkinsonism Relat Disord 16:79–84
Gelpi E, Navarro-Otano J, Tolosa E, Gaig C, Compta Y, Rey MJ, Martí MJ, Hernández I, Valldeoriola F, Reñé R, Ribalta T (2014) Multiple organ involvement by alpha-synuclein pathology in Lewy body disorders. Mov Disord 29:1010–1018
Yan D, Zhang Y, Liu L, Shi N, Yan H (2018) Pesticide exposure and risk of Parkinson’s disease: dose-response meta-analysis of observational studies. Regul Toxicol Pharmacol 96:57–63
McCann H, Cartwright H, Halliday GM (2016) Neuropathology of α-synuclein propagation and braak hypothesis. Mov Disord 31:152–160
Felice VD, Quigley EM, Sullivan AM, O’Keeffe GW, O’Mahony SM (2016) Microbiota-gut-brain signalling in Parkinson’s disease: implications for non-motor symptoms. Parkinsonism Relat Disord 27:1–8
Pan-Montojo F, Anichtchik O, Dening Y, Knels L, Pursche S, Jung R, Jackson S, Gille G, Spillantini MG, Reichmann H, Funk RH (2010) Progression of Parkinson’s disease pathology is reproduced by intragastric administration of rotenone in mice. PLoS One 5:e8762
Svensson E, Horváth-Puhó E, Thomsen RW, Djurhuus JC, Pedersen L, Borghammer P, Sørensen HT (2015) Vagotomy and subsequent risk of Parkinson’s disease. Ann Neurol 78:522–529
Gilman S, Wenning GK, Low PA, Brooks DJ, Mathias CJ, Trojanowski JQ, Wood NW, Colosimo C, Dürr A, Fowler CJ, Kaufmann H, Klockgether T, Lees A, Poewe W, Quinn N, Revesz T, Robertson D, Sandroni P, Seppi K, Vidailhet M (2008) Second consensus statement on the diagnosis of multiple system atrophy. Neurology 71:670–676
Kikuchi A, Takeda A, Okamura N, Tashiro M, Hasegawa T, Furumoto S, Kobayashi M, Sugeno N, Baba T, Miki Y, Mori F, Wakabayashi K, Funaki Y, Iwata R, Takahashi S, Fukuda H, Arai H, Kudo Y, Yanai K, Itoyama Y (2010) In vivo visualization of alpha-synuclein deposition by carbon-11-labelled 2-[2-(2-dimethylaminothiazol-5-yl)ethenyl]-6-[2-(fluoro)ethoxy] benzoxazole positron emission tomography in multiple system atrophy. Brain 133:1772–1778
Tateno F, Sakakibara R, Kawai T, Kishi M, Murano T (2012) Alpha-synuclein in the cerebrospinal fluid differentiates synucleinopathies (Parkinson disease, dementia with Lewy bodies, multiple system atrophy) from Alzheimer disease. Alzheimer Dis Assoc Disord 26:213–216
Sakakibara R, Panicker JN, Aiba Y, Tateno F, Ogata T, Yano M, Suzuki H, Sugisaki Y, Shimizu A, Takahashi O, Simeoni S (2020) Possible “premotor” multiple system atrophy-cerebellar form. Eur Neurol 83:80–86
Sakakibara R, Odaka T, Uchiyama T, Liu R, Asahina M, Yamaguchi K, Yamaguchi T, Yamanishi T, Hattori T (2004) Colonic transit time, sphincter EMG, and rectoanal videomanometry in multiple system atrophy. Mov Disord 19:924–929
Yamamoto T, Sakakibara R, Uchiyama T, Yamaguchi C, Nomura F, Ito T, Yanagisawa M, Yano M, Awa Y, Yamanishi T, Hattori T, Kuwabara S (2012) Receiver operating characteristic analysis of sphincter electromyography for parkinsonian syndrome. Neurourol Urodyn 31:1128–1134
Yamamoto T, Tateno F, Sakakibara R, Furukawa S, Asahina M, Uchiyama T, Hirano S, Yamanaka Y, Fuse M, Koga Y, Yanagisawa M, Kuwabara S (2016) Urinary dysfunction in progressive supranuclear palsy compared with other parkinsonian disorders. PLoS One 11(2):e0149278. https://doi.org/10.1371/journal.pone.0149278
Doi H, Sakakibara R, Tateno F, Aiba Y (2020) Colonic transit time in progressive supranuclear palsy and Parkinson’s disease. https://doi.org/10.1111/ncn3.12473
Kobal J, Matej K, Koželj M, Podnar S (2018) Anorectal dysfunction in presymptomatic mutation carriers and patients with Huntington’s disease. J Huntingt Dis 7:259–267
Aldaz T, Nigro P, Sánchez-Gómez A, Painous C, Planellas L, Santacruz P, Cámara A, Compta Y, Valldeoriola F, Martí MJ, Muñoz E (2019) Non-motor symptoms in Huntington’s disease: a comparative study with Parkinson’s disease. J Neurol 266:1340–1350
Suttrup I, Suttrup J, Suntrup-Krueger S, Siemer ML, Bauer J, Hamacher C, Oelenberg S, Domagk D, Dziewas R, Warnecke T 2017 Esophageal dysfunction in different stages of Parkinson’s disease. Neurogastroenterol Motil 29(1). https://doi.org/10.1111/nmo.12915
Ueha R, Goto T, Sato T, Nativ-Zeltzer N, Shen SC, Nito T, Belafsky PC, Yamasoba T (2018) High resolution manofluorographic study in patients with multiple system atrophy: possible early detection of upper esophageal sphincter and proximal esophageal abnormality. Front Med (Lausanne) 5:286. https://doi.org/10.3389/fmed.2018.00286 eCollection 2018
Claus I, Suttrup J, Muhle P, Suntrup-Krueger S, Siemer ML, Lenze F, Dziewas R, Warnecke T (2018) Subtle esophageal motility alterations in parkinsonian syndromes: synucleinopathies vs. tauopathies. Mov Disord Clin Pract 5:406–412
Sakakibara Y, Asahina M, Suzuki A, Hattori T (2009) Gastric myoelectrical differences between Parkinson’s disease and multiple system atrophy. Mov Disord 24:1579–1586
Krygowska-Wajs A, Furgala A, Gorecka-Mazur A, Pietraszko W, Thor P, Potasz-Kulikowska K, Moskala M (2016) The effect of subthalamic deep brain stimulation on gastric motility in Parkinson’s disease. Parkinsonism Relat Disord 26:35–40
Thomaides T, Karapanayiotides T, Zoukos Y, Haeropoulos C, Kerezoudi E, Demacopoulos N, Floodas G, Papageorgiou E, Armakola F, Thomopoulos Y, Zaloni I (2005) Gastric emptying after semi-solid food in multiple system atrophy and Parkinson disease. J Neurol 252:1055–1059
Barnett WH, Jenkin SEM, Milsom WK, Paton JFR, Abdala AP, Molkov YI, Zoccal DB (2018) The Kölliker-Fuse nucleus orchestrates the timing of expiratory abdominal nerve bursting. J Neurophysiol 119:401–412
Borders JC, Brandimore AE, Troche MS (2020) Variability of voluntary cough airflow in healthy adults and Parkinson’s disease. Dysphagia. https://doi.org/10.1007/s00455-020-10190-3
Mathers SE, Kempster PA, Swash M, Lees AJ (1988) Constipation and paradoxical puborectalis contraction in anismus and Parkinson’s disease; a dystonic phenomenon? J Neurol Neurosurg Psychiatry 51:1503–1507
Tateno F, Sakakibara R, Yokoi Y, Kishi M, Ogawa E, Uchiyama T, Yamamoto T, Yamanishi T, Takahashi O (2011) Levodopa ameliorated anorectal constipation in de novo Parkinson’s disease: the QL-GAT study. Parkinsonism Relat Disord 17:662–666
Tateno H, Sakakibara R, Shiina S, Doi H, Tateno F, Sato M, Masaka T, Kishi M, Tsuyusaki Y, Aiba Y, Ogata T, Suzuki Y (2015) Transdermal dopamine agonist ameliorates gastric emptying in Parkinson’s disease. J Am Geriatr Soc 63:2416–2418
Rao SS, Beaty J, Chamberlain M, Lambert PG, Gisolfi C (1999) Effects of acute graded exercise on human colonic motility. Am J Phys 276(5 Pt 1):G1221–G1226
Sakakibara R, Tsunoyama K, Hosoi H, Takahashi O, Sugiyama M, Kishi M, Ogawa E, Terada H, Uchiyama T, Yamanishi T (2010) Influence of body position on defecation in humans. Lower Urin Tract Symptoms 2:16–21
Zangaglia R, Martignoni E, Glorioso M, Ossola M, Riboldazzi G, Calandrella D, Brunetti G, Pacchetti C (2007) Macrogol for the treatment of constipation in Parkinson’s disease. A randomized placebo-controlled study. Mov Disord 22:1239–1244
Sakakibara R, Yamaguchi T, Uchiyama T, Yamamoto T, Ito T, Liu Z, Odaka T, Yamaguchi C, Hattori T (2007) Calcium polycarbophil improves constipation in primary autonomic failure and multiple system atrophy subjects. Mov Disord 22:1672–1673
Ondo WG, Kenney C, Sullivan K, Davidson A, Hunter C, Jahan I, McCombs A, Miller A, Zesiewicz TA (2012) Placebo-controlled trial of lubiprostone for constipation associated with Parkinson disease. Neurology 78:1650–1654
Freitas ME, Alqaraawi A, Lang AE, Liu LWC (2018) Linaclotide and prucalopride for management of constipation in patients with parkinsonism. Mov Disord Clin Pract 5:218–220
Tan AH, Lim SY, Chong KK, Azhan A Manap MA, Hor JW, Lim JL, Low SC, Chong CW, Mahadeva S, Lang AE (2020) Probiotics for constipation in Parkinson’s disease: a randomized placebo-controlled study. Neurology. https://doi.org/10.1212/WNL.0000000000010998.
Liu Z, Sakakibara R, Odaka T, Uchiyama T, Uchiyama T, Yamamoto T, Ito T, Asahina M, Yamaguchi K, Yamaguchi T, Hattori T (2005) Mosapride citrate, a novel 5-HT4 agonist and partial 5-HT3 antagonist, ameliorates constipation in parkinsonian patients. Mov Disord 20:680–686
Sullivan KL, Staffetti JF, Hauser RA, Dunne PB, Zesiewicz TA (2006) Tegaserod (Zelnorm) for the treatment of constipation in Parkinson’s disease. Mov Disord 21:115–116
Sakakibara R, Doi H, Sato M, Hirai S, Masaka T, Kishi M, Tsuyusaki Y, Tateno A, Tateno F, Aiba Y, Ogata T, Suzuki Y (2015) Nizatidine ameliorates slow transit constipation in Parkinson’s disease. J Am Geriatr Soc 63:399–401
Sakakibara R, Odaka T, Lui Z, Uchiyama T, Yamaguchi K, Yamaguchi T, Asahina M, Yamamoto T, Ito T, Hattori T (2005) Dietary herb extract dai-kenchu-to ameliorates constipation in parkinsonian patients (Parkinson’s disease and multiple system atrophy). Mov Disord 20:261–262
Doi H, Sakakibara R, Sato M, Hirai S, Masaka T, Kishi M, Tsuyusaki Y, Tateno A, Tateno F, Takahashi O, Ogata T (2014) Dietary herb extract rikkunshi-to ameliorates gastroparesis in Parkinson’s disease: a pilot study. Eur Neurol 71:193–195
Malhotra A, Shah N, Depasquale J, Baddoura W, Spira R, Rector T (2016) Use of Bristol Stool Form Scale to predict the adequacy of bowel preparation – a prospective study. Color Dis 18:200–204
Cadeddu F, Bentivoglio AR, Brandara F, Marniga G, Brisinda G, Maria G (2005) Outlet type constipation in Parkinson’s disease: results of botulinum toxin treatment. Aliment Pharmacol Ther 22:997–1003
Daeze C, Van Biervliet S, Van Laecke E, Van Winckel M, De Bruyne R, De Guchtenaere A, Hoebeke P, Vande VS (2018) The predictive value of colon transit time and anorectal manometry in the approach of faecal continence in children with spina bifida. Acta Gastroenterol Belg 81:277–282
Lacima G, Pera M, González-Argenté X, Torrents A, Valls-Solé J, Espuña-Pons M (2016) Is electromyography a predictive test of patient response to biofeedback in the treatment of fecal incontinence? Neurourol Urodyn 35:390–394
Kollmann CT, Pretzsch EB, Kunz A, Isbert C, Krajinovic K, Reibetanz J, Kim M (2020) Anorectal angle at rest predicting successful sacral nerve stimulation in idiopathic fecal incontinence—a cohort analysis. Int J Color Dis 35:2293–2299
Ogawa E, Sakakibara R, Kishi M, Tateno F (2012) Constipation triggered the malignant syndrome in Parkinson’s disease. Neurol Sci 33:347–350
Funding
The authors did not receive support from any organization for the submitted work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
This article followed standard ethical consideration and was prepared according to the Declaration of Helsinki. Ethical approval was waived by the Local Ethics Committee in view of the retrospective nature of the study.
Conflict of interest
The authors declare that they have no conflict of interest.
Informed consent
None required because this is a review article.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sakakibara, R. Gastrointestinal dysfunction in movement disorders. Neurol Sci 42, 1355–1365 (2021). https://doi.org/10.1007/s10072-021-05041-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-021-05041-4