Abstract
Aluminum (Al) is used in packaging due to its density, strength, and preservative qualities. The Norwegian packaging flows in 2020 are shown. The paper outlines a laboratory methodology to evaluate the recycling performance of Al packaging where parameters of collection type and pre-treatment are alternated with measurement of yield and analysis of metal quality. Further, the paper evaluates the recyclability of the Al from (1) sorted at a materials recovery facility for municipal waste, (2) from a co-mingled glass and metal packaging collection system, (3) used beverage cans from a deposit system, and (4) from a novel collection scheme. The benefit of pre-treatment is evaluated for all these systems. The results indicate acceptable yield and metal quality from remelting in all four collections systems, but care should be taken in the comparison. Collection, pre-treatment, and melting processes need to be assessed together. Overall, thermal treatment at 550°C is better than at 300°C, which is also worse than just drying at 110°C. This study aims to contribute to assessing Al packaging recycling in Norway and help future developments.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Al is often applied in packaging due to its formability, strength, and protective quality. Food and beverage are fast-moving consumer products with a relatively short lifetime, and mostly non-reusable. This results in a large and continuous flow of end-of-life (EoL) Al packaging waste, which should be recycled according to circular economic principles.
However, it is challenging to recycle Al packaging due to its thickness, organic residue, water, and hydrocarbon coatings (the latter three referred to as ‘contamination’). Al packaging often consists of very thin Al layers. Classical studies performed by Rossel1 have demonstrated the role of the thickness on metal loss. The thinner the scrap, the higher the metal loss, in particular for gauge thickness under 2 mm. Thermal decoating, where the waste is heated in the presence of oxygen, reduces the contamination and thus increases yield. In a study,2 organic residue from food had a larger effect on yield than the coatings.
At present, the recycling of Al packaging in Norway takes place through three main schemes defined by the point of collection.3 These three schemes are shown in Fig. 1 and described in the text after the figure. It can be seen that the mass flows of the used beverage cans (UBC) (turquoise and pink arrows) are far greater than food packaging (blue arrows), where the domestic UBC makes up about 70 mass% of the total EoL Al packaging and imported UBC, about 22%, and food packaging only 8%. Recent numbers for the beverage deposit system show that, in 2021, 91.5% of UBC had been collected out of 1.03 billion UBC,4 which is 61% of all recycled cans and bottles with a deposit–refund system. The rest are polyethylene terephthalate bottles which are also a part of the deposit return scheme.
The mass flow of Al packaging in Norway in 2020. The figure is a simplification of the flow shown in the report.3 This figure is reprinted with permission from SINTEF. See text for explanation of A, B, and C (Color figure online).
As shown in Fig. 1A, municipal household residual waste that contains Al, some of the Al used in packaging materials end up in the residual household waste bins either due to incorrect sorting or because the consumer is instructed to sort the packaging as residual waste, such as household foil and coffee capsules. The misplacement could simply also be because of a large amount of food residue, or lack of a convenient collection system for metal packaging. After sorting, most residual household waste in Norway is sent directly to incineration. Some Al can be recovered by sifting it from the bottom ash produced during the incineration. The recovery rate of Al through incineration plants in Norway is estimated to be 62%.5 The loss of Al during incineration is difficult to measure, and the number should only be considered as an approximation. The sifted Al is valuable and can be sorted by size and alloy and sold on the scrap market.
As shown in Fig. 1B, municipal collection systems glass and metal packaging co-mingle bin, bins for glass and metal packaging are placed at households or public collection points throughout the municipalities in Norway. At a central sorting facility, metal and non-metallic materials (mainly glass) are separated, and then the metal is further split into ferrous (magnetic) and non-ferrous (non-magnetic) metal. The sorted Al fraction from the non-ferrous metal is sent to scrap dealers for pressing, and then further to remelting. This Al is often used in foundry alloys.
As shown in Fig. 1C, deposit–refund recycling for used beverage cans (UBC), the consumer pays a small deposit when buying the beverage (both Al and plastic) and is refunded when returning the empty container through reverse vending machines in supermarkets or at manual return points in smaller shops or kiosks. Al cans are separated out and baled. Pressed UBC consists of almost pure Al, has a consistent quality, and is available in large quantities. It is a valuable commodity and traded at indexed prices. The baled UBCs are remelted in a reverberatory or rotary furnace, the latter often with salt flux and the former often with pre-heating. Wrought Al and rolled products are the main recycled outcomes, sometimes used as input to production of new Al cans.
Pick analyses from 2020 showed that the co-mingled glass and metal packaging waste contains 9% EoL Al packaging with about 10% organic contamination.6 Pick analyses from 2022 showed that 8% of EoL Al packaging in Norway ended up in incineration.7
A laboratory method for evaluating the quality and yield of the Al fraction from Norwegian Material Recovery Facilities (MRFs) has been reported previously.8 Recycling of Al food tubes was studied by the same group and was presented at the TMS conference in San Diego in 2023.2
The work presented in this paper focuses on laboratory-scale recycling of EoL packaging in the three schemes mentioned above, plus a pilot test of Al packaging from home delivery customers. The paper also discusses tests from scheme B, glass, and metal on an industrial scale.
Better sorting is one of the core topics for the improvement of Al packaging recycling in general. Actions in sorting can involve sorting technology, i.e., MRFs and sorting by the consumer. The change in waste labelingFootnote 1 introduced in Norway in 2020 has made it easier for the customer to recycle efficiently. Norway has built MRFsFootnote 2 from 2014, which sort the household waste into different materials fractions to enable further treatment, ideally more materials recycling. The advantage of this technological solution somewhat reduces the dependence on customer sorting. MRFs have become more popular for waste management because of the high recovery of secondary raw materials, such as organic, plastics, and paper.9 However, the MRFs operating in Norway have major issues with contamination of fractions sorted from the household waste. Especially, the food residues on paper, etc. is very harmful. This has been a costly learning experience for the MRFs. Major problems are that large municipalities do not yet separate out food waste. Packaging, labeled to be thrown in residual waste or not designed to be recycled, are sorted for incineration. MRFs are also associated with high costs because the construction and operation of such systems is quite complex and involves the use of a wide range of technologies. Based on the work in Austria,10 the installation of MRFs is not a substitute for customer sorting of Al packaging.
To meet recycling targets set by legislation and to be resource efficient, the plan is to build more MRFs in Norway. The discussion is how advanced these should be to balance the cost versus yield/quality of the various materials. Placing an additional bin for metal next to the other household bins has shown a tendency to increase the collected metal. Therefore, a trend of picking up metal at the house is expected. The deposit return system is a proven scheme dating from the 1980s that has inspired many countries. For instance, Scotland recently decided to enforce it. Also, many of the states in the USA have deposit return systems.
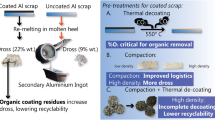
Small amounts of coating are necessary for Al packaging, but a lot of hydrocarbon lacquering, etc. is applied to improve marketability. This challenges the recycling process. If the coating is not removed before remelting, it can reduce the recycling yield.11 The fundamental process of decoating is described in Ref. 12. Even though thermal treatment is a standard industrial practice for decoating,13 burning of the coatings can increase oxidation and lead to environmentally unfriendly off-gas.14 Oxidation can lead to metal loss and dross formation. Compaction15,16 has been suggested as a method for preventing oxidation by reducing the surface area.17 The proper temperature and time for thermal treatment is critical. Al waste is often dried to evaporate water for security preventing molten metal explosions. De-coating kilns are usually run at 550–570°C (with a few % O2 surplus). In practice, during de-coating, the metals are underexposed due to differences in scrap morphology, too low residence times, surfaces not exposed to the atmosphere, etc. Visually, this is seen by the metal surfaces being “black”, meaning there are carbon residues left on them. As in a previous study,8 110°C, 300°C, and 550°C have been chosen as the thermal treatment temperatures in the current work to simulate drying, under decoating, and optimum decoating.
Al scrap can be remelted in a reverberatory furnace, a rotary furnace, a crucible, or in electrical furnaces in industry.18,19 The thermodynamic barrier for Al to the removal of most elements is quite large according to the Ellingham diagram. Only magnesium (Mg) and calcium (Ca) can be effectively removed from the melt by simple oxidation. In practice, fluxes are useful in removing Ca, Mg, sodium (Na), etc. from Al by forming more stable chlorides and fluorides than Al, which can then be removed from the melt. A salt layer can protect the metal underneath from further oxidation. Impurities, inclusions, and oxides can be extracted from the melt and the extracted metal entrapped in the dross. Fluorides are often added to the salt to enhance coalescence. Fluorides help strip and break up the oxide layers so that imprisoned metal droplets can be accessed and coalescence improved.20,21 Even though salt treatment is therefore attractive, it is environmentally more and more non-acceptable because of the production of salt slags,22 the cost, and safety hazards.23,24 In this work, EoL Al was remelted by submersion into a pure molten Al heel. The purpose is not dilution, rather an approach to recycling EoL Al packaging in a more environmentally friendly and cost-effective way. Industrially, one or more processing steps may be needed afterwards, for example, adjusting the composition and removing some critical elements.
Experimental
This work focuses on recycling of EoL Al packaging on a laboratory scale (1–4) and on an industrial scale (5) as following:
-
1.
Municipal waste
Al packaging, such as food and beverage containers, small pieces of Al attached onto larger waste materials, and laminated and multilayer Al packaging from a mixed household waste MRF sorting plant was received. In addition, our samples were hand-picked at the MRF in order to remove aerosol cans and some other contaminants (e.g., pads underneath fish and meat), but leaving most of the material in to make it more representative.
-
2.
Co-mingled glass and metal packaging waste
Pressed material from a Norwegian plant (NMG) was received from their sorting plant. Even though the sorting line has since been rebuilt, it is believed that the material in the test is also representative for today’s Al scrap. The pressed material was cut into cubes after it was received. One can clearly see glass pieces in the Al.
-
3.
UBC
Compressed UBC was received. This originated from the deposit–refund system. This material was also cut into appropriately sized pieces before the experiment.
-
4.
Supermarket pilot
Customers of a supermarket chain that offers home delivery of food and groceries in Norway were given bags in to which the customers were asked to put metal that would be picked up at the next food home delivery. A total of 42 bags were returned and manually sorted into 5 groups: non-magnetic metal, magnetic metal, glass, plastic, and other. The non-magnetic metal was further sorted into 9 groups, as listed in the horizontal axis in Fig. 2.
As shown in Table I, all the Al scrap had been compacted either by us (sources 1 and 4) at 35 MPa in an industrial metal press or as received (2 and 3). According to the practical cases, various preheating temperatures were chosen: the municipal waste was preheated at 300°C and 550°C, since it is generally incinerated without drying. In the pilot trials, it is only dried due to the limited volume of the materials. With enough materials, NMG glass and metal were preheated at 110, 300, and 550°C to be compared with the rest. The materials for the laboratory experiments were cut into cubes, preheated in an electrically heated box furnace in air, and then remelted in an Al heel at 750°C with 4 times scrap addition. The amount of Al heel was adjusted due to the amount of scrap, since at least 5 kg metal was required to heat evenly in the custom-made drop coil induction furnace. The dross was skimmed after each addition. To examine the metal quality, the following were carried out: a reduced pressure test (RPT)25 to analyze the porosity of a solidified Al sample, porous disk filtration analysis (PoDFA)26 to characterize inclusion types and their area, and optical emission spectrometry (OES) to determine the chemical compositions of the metal for all tests.
-
5.
Industrial remelting in rotary furnace
Metallco started to remelt the Al fraction of food containers from co-mingled glass and metal bins from 2020. For this study, 7 batches of 3 tonnes each of the Al fraction sorted from co-mingled glass and metal bins were shred, preheated, and remelted with salt in a rotary furnace.
Results and Discussion
Sorting
The sorting of source 1 (municipal waste) was carried out both automatically by the MRF and thereafter hand-sorted in the laboratory. An initial test showed that a fraction, a mix of Al foil and multimaterials, lost 84% of its mass during decoating, and therefore also had a lot of ash trapped in the remaining decoated metal. Thus, this Al foil was not suitable for remelting in an Al heel in the laboratory setup. Around 50% of the received material from source 1 was sorted out for this reason.
Sources 2 (glass and metal) and 3 (UBC) had been sorted automatically by two Norwegian plants. The hand sorting in the laboratory showed that, in source 4 (supermarket pilot) 47% was glass, and the other 52% was metal. It can be noted that, in the instructions, only metals were asked for. The metal fraction was 42% magnetic metals and 10% non-magnetic metals (mostly Al and some ‘surprises’, such as silver plating, tin, and bronze, etc.) in a total of 85.1 kg co-mingled glass and metal packaging waste. The remaining 1% was non-glass and non-metal. As shown in Fig. 2, Al food cans and their lids, tealights, UBC, and Al food tubes added up to 78% of all non-magnetic metals collected by the pilot. Surprisingly, the tealight amount was much higher than the average tealight waste in source 2 (0.1%), which is believed to be more representative. Aerosol cans can be dangerous when being remelted, partly depending on their contents, such as pesticides, and the risk of explosion. These cans should be collected separately, and therefore any present in our sample materials were removed.
From a metallurgical point of view, the metal yield after remelting is vital. As stated in the Introduction, contaminated, coated materials reduce the yield. Thus, the Al fraction in the pilot was further grouped into clean and with/without coating, 5.1 kg (cans, UBC, and containers with coating, household foil, containers and trays without coating), and laminated and dirty, 3.5 kg (tealights with wax, tubes, laminated foil and others). Then, the laminated and dirty were further labeled as dirty scrap and the other as clean scrap.
Sorting is one of the major issues in Al packaging recycling. Two of the four collection systems in the current work were sorted by the customers. Hand-sorting at the laboratory was carried out for the supermarket pilot.
Thermal Treatment
The pre-treatment from all four collection sources is shown in Fig. 3 for the thermal treatment temperatures of 110°C, 300°C, and 550°C. The scrap yield is defined as:
The scrap yield of the municipal Al packaging waste was close to 60% and much lower than that of the UBC (96%), pilot (79%) and NMG glass with metal co-mingle (96–99%). The pilot had a higher scrap yield than that from the municipal waste, but less than from NMG. As expected, the UBC had the largest yield due to the minimum beverage residue. The yield reflects the average coatings on the UBC of around 3–4% coatings. The weight loss from the pilot was mainly due to the organic residue, which after thermal treatment looks like fat and wax (Fig. 4).
Remelting
Remelting Yield
The remelting yield can be defined as
To help in the comparison of yields from various scrap sources with various amounts of Al heel and scrap addition, a yield based on scrap addition has also been defined, assuming the Al heel does not react or produce dross during remelting.
After pre-treatment, the scrap was remelted, as described in the experimental section. The ratio between the scrap and the heel varies for sources 1–4. Figure 5a shows the absolute yield. The pilot gave the greatest yield (72%), while the NMG yield was the lowest (37–45%).
Figure 5b shows the scrap-based yield. Here, we see that NMG produces negative yield, which means that the metal produced from the scrap is less than the Al heel. This illustrates that this scrap metal in particular interacts with the heel, indicating that the old rule in the recycling industry that for every 1% of contamination charges into the melting furnace, there will be at least an equivalent 1% metal loss. It also explains the low metal yield of NMG in Fig. 5a. For glass and metal, The NMG (automatically sorted in industry) gives much less yield than the pilot (hand sorted), showing that hand sorting gives a better yield. The scrap-based yield is quite similar for the municipal waste, UBC and pilot, 42–54%. Generally, one can expect the yield would increase for Al recycling from municipal waste compared to glass and metal bins and UBC considering the cleanliness and gauge thickness with the same practice. These results show that all three sources can achieve relatively comparable yields with improved sorting.
Figure 5a and b illustrate that, overall, thermal treatment at 550°C gives a better recycling yield than at 300°C, Also the recycling yield is less at 300°C than just drying at 110°C. This is in accordance with the results of another study.18 The recycling yield for Al food tubes was 34–58% in salt in a previous work.2 These tubes in general will end up either in glass and metal bins or as municipal household waste, which fits in the recycling yield range of the current work.
Remelting Quality
In Fig. 6, the bifilm index from the collection sources 1–4 is shown together with its uncertainty. The bifilm index is the total length of bifilms on the sectioned surface of the RPT sample,27 with a high number indicating low quality and vice versa. Figure 6 indicates that pre-treatment at 550°C increases the metal quality. With the same metal/scrap ratio, the pilot has not only a higher yield but also less oxide films than NMG. This indicates that the Al fraction in glass and metal co-mingled waste from bins are much dirtier or mixed with other nonmetals than the stream collected directly from the pilot customers. One possible reason is that hand sorting in the laboratory is more beneficial than machine sorting, even though it is not efficient. This calls for better home sorting habits for consumers.
PoDFA measures the area of mixed oxide, carbide, and TiB2/Ti-rich particles in a filtered sample. In Fig. 7, the total particles from sources 1–4 are shown as absolute values with various metal and scrap additions (Table I). With the same metal/scrap ratio, the supermarket pilot gives less bifilms but more inclusions than NMG. We see that the heat treatment at 550°C does not seem to give any advantage, especially for municipal waste and UBC. Also, it gives the opposite trend of the RPT (except for NMG), indicating that these total particles are not bifilms. Thus, the supermarket pilot contains relatively high amounts of particles, but small amounts of bifilms, while UBC contains relatively high amounts of both particles and bifilms. UBC and the pilot have higher total particles than municipal waste. Thermodynamically, the pure molten Al heel in the current work is not as favorable as the salt flux.
Filtration, fluxing, floatation, and sedimentation can contribute to the removal of the inclusions. However, the aim of the current work is not refining but to outline a laboratory methodology to evaluate the recycling performance of Al packaging, and to evaluate the recyclability of the Al waste from Al packaging schemes in Norway.
Chemical Composition
Figure 8 shows the chemical compositions, measured by standard OES, of the alloying and trace elements for all the collection schemes in wt.% and ppm. These compositions are diluted due to the mixture of Al heel and scrap. Volatile elements, such as magnesium (Mg) and zinc (Zn), are affected by pre-treatment temperatures, and therefore have a relatively large standard deviation from the same collection scheme when presented together. However, the variation also reflects that scrap is non-uniform, while UBC is more uniform than NMG, as expected. Vanadium (V), antimony (Sb), gallium (Ga), and silver (Ag) are generally high as minor elements for NMG. The large variation in Ag is, for instance, suspected to come from misplacing silver ware or plating in the recycling scheme of NMG. UBC contains larger Sb, while municipal waste contains larger Ga. The pilot contains very low amounts of almost all the elements except Fe, thanks to the direct collection and strict hand sorting, and of course more dilution with the Al heel. This indicates that good sorting directly contributes to the stable composition in the final product. The variance reflects the different alloys used in UBC (typically 0.9% Mn and 1.95% Mg13) and food packaging (1xxx and 8xxx alloys). Remelting generally achieves the composition specification limits of both wrought and cast alloys. However, some elements need to be diluted, as well as some main elements need to be added.
Remelting Yield and Quality of Source 5 Industry
Remelting of Al packaging from scheme B, glass and metal bins in an industrial trial, gave generally low Si and Mg, as shown in Table II while it yielded abnormally high contents of Zn and Sn. The origin of the Zn and Sn is unknown. One proposed source could be Fe packaging that was not sorted out through the magnetic separation, which often contain Sn and/or Zn plating. However, the amounts cannot explain this high composition. Other sources may be the coatings on the packaging, or the presence of pure Sn and Zn packaging or scrap, or Sn caps on glass bottles. To obtain the target AC46000 alloy, Si, Cu, Fe, and Mg should be added. Also, Zn and Sn should be diluted with pure Al, as there is no economical known method to remove them. Most of the elements in the industrial case (Table II) are in the same range as that of glass and metal in scheme B, NMG and the pilot (Fig. 8). The data in Fig. 8 are from experiments with diluted melts, around 50% dilution. As expected, salt fluxing results in a much lower Mg concentration. The scrap-based yield is 55% for this industrial case, which is similar to the supermarket pilot. Thus, hand sorting and salt treatment are both favorable from the aspect of yield.
The Recycling Chains
The current work provides insights into the Al packaging recycling chains of collection, sorting, pre-treatment, and remelting in Norway.
It shows that hand sorting has the advantage of better yield, even though it is not as effective as an MRF. It points to the importance of increasing the sorting awareness of the public. Meanwhile, the cost versus yield/quality should be balanced for more advanced MRFs.
Al scrap from scheme A (municipal waste) often lost more than 40% weight during pre-treatment due to the organic residue and lamination. Scrap from scheme B (glass and metal) and C (UBC) lost less than 20% weight. since they are generally cleaner than that from scheme A.
According to Figs. 5, 6, and 7, the pilot and UBC gave the highest yield but lowest quality. One explanation can be that removing the dross means less yield and less inclusions.
Conclusion
The collection of EoL Al packaging waste in Norway is managed through three schemes: (A) municipal household residual waste collection, (B) municipal collection of glass and metal co-mingled waste, and (C) deposit–refund systems. This scrap has been recycled and remelted in an Al heel on the laboratory scale, and in salt in an industrial rotary furnace, and the results are presented in the current work.
This study shows that EoL Al packaging can be recycled with acceptable yield and metal quality in an Al heel. The scrap yield from thermal pre-treatment varies from 56% to 99%, while the recycling yield varies from 32% to 80%. A scrap-based yield was also defined, illustrating that contamination generated more metal loss. The thermal treatment at 550°C is better than at 300°C. Drying at 110°C often removes any organic food residue.
The chemistry is easily distorted with poor sorting. For example, the industrial trial from remelting from scheme B (glass and metal) gave high levels of Zn and Sn. This material would have to be diluted to obtain the product requirements.
Material recovery facilities in Norway are not yet sufficient to meet higher quality and yield targets. Better or additional sorting and pre-treatment are required.
Notes
Ny merkeordning klar til å tas i bruk! - Avfall Norge (New branding scheme ready to be used!—Waste Norway).
References
H. Rossel, Light Met. 1990, 721 (1990).
S. Bao, A. Kvithyld, G.A. Bjørlykke, and K. Sandaunet, Light Metals (Springer Nature, New York, 2023), pp. 960–966.
M. Skramstad, M. Syvertsen, and A. Kvithyld, Aluminium Packaging Flow in Norway, Report (SINTEF, Norway, 2021).
INFINITUM, Annual Report (2021).
R. Briedis, Gjennvinning av metaller fra forbrenningsaske (2018).
S. Bjørnerud, Plukkanalyser glass/metall (2020).
B. Malcolm, Plukkanalyser 2022, Presented at project meeting of Alpakka (2023).
S. Eggen, K. Sandaunet, L. Kolbeinsen, and A. Kvithyld, Light Metals (Springer Nature, New York, 2020), pp. 1091–1100.
M.A. Budihardjo, S.Y. Ardiansyah, and B.S. Ramadan, Habitat Int. 119, 102488 https://doi.org/10.1016/j.habitatint.2021.102488 (2022).
R. Warrings, and J. Fellner, Waste Manag. Res. https://doi.org/10.1177/0734242X20947161 (2021).
S. Capuzzi, A. Kvithyld, G. Timelli, A. Nordmark, E. Gumbmann, and T.A. Engh, J. Sustain. Metall. 4, 343358 (2018).
A. Kvithyld, C.E.M. Meskers, S. Gaal, M. Reuter, and T.A. Engh, JOM 60, 47 (2008).
T.A. Engh, G.K. Sigworth, and A. Kvithyld, Principles of Metal Refining and Recycling (Oxford University Press, Oxford, 2021), pp611–637.
S. Høgåsen, The Effect of Compaction and Thermal Treatment in the Recovery of Coated Aluminium Scrap Through FTIR Off-Gas Analysis and Remelting in Molten Heel (Norwegian University of Science and technology, Trondheim, 2022), pp18–30.
A. Vallejo-Olivares, S. Høgåsen, A. Kvithyld, and G. Tranell, Light Metals (Springer, New York, 2022), pp. 1029–1037.
A. Vallejo-Olivares, S. Høgåsen, A. Kvithyld, and G. Tranell, J. Sustain. Metall. 8, 1485 (2022).
C. Hamers and A. Jessberger, “Aluminium Cycle: Machining, Briquetting, Melting” (Global Recycling, 2018). https://global-recycling.info/archives/2354. Accessed 10 August 2022.
S. Capuzzi, and G. Timelli, Metals. https://doi.org/10.3390/met8040249 (2018).
C. Schmitz, Handbook of Aluminium Recycling: Mechanical Preparation, Metallurgical Processing, Heat Treatment (Vulkan-Verlag, Essen, Germany, 2014), pp185–274.
M.S.S. Besson, A. Pichat, E. Xolin, P. Chartrand, and B. Friedrich, in Proceedings of EMC (2001), pp. 1–26.
R. D. Peterson, in Proceedings of the 2th International Symposium on Recycling of Metals and Engineered Materials, 69 (1990).
N. Ünlü, and M.G. Drouet, Resour. Conserv. Recycl. 36, 61 (2002).
A. Srivastava, and A. Meshram, Process. Saf. Environ. Prot. 171, 38 https://doi.org/10.1016/j.psep.2023.01.010 (2023).
A. Meshram, R. Jha, and S. Varghese, Mater. Today Proc. 46, 1487 (2021).
D. Dispinar, Metal Quality of Aluminium and Its Alloys (The University of Birmingham, Birmingham, 2005).
C. Stanica, and P. Moldovan, UPB Sci. Bull. Ser. B Chem. Mater. Sci. 71, 107 (2009).
D. Dispinar, and J. Campbell, Int. J. Cast Met. Res. 19, 5 (2006).
Acknowledgements
This research was carried out as part of the Norwegian Research Council (NRC)—funded IPN Project (296276) Alpakka-Circular Al Packaging in Norway. Funding by the industrial partners and NRC is gratefully acknowledged. Thanks are also given to Norsk metallgjenvinning, Metallco Al, Norsk Hydro, Infinitum, Meny, IVAR IKS for support, supplying scrap and insight.
Funding
Open access funding provided by SINTEF.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bao, S., Eggen, S., Syvertsen, M. et al. Collection, Thermal Treatment, and Remelting End-of-Life Al Packaging in Norway. JOM 75, 5755–5763 (2023). https://doi.org/10.1007/s11837-023-06195-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11837-023-06195-6