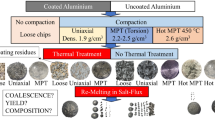
Abstract
Organic coatings are a challenge for aluminium packaging recycling since they tend to increase the re-melting metal losses. A solution is de-coating the scrap via a thermal pre-treatment to burn-off the organics before re-melting. Due to logistic benefits, the scrap is often pressed into bales. This study evaluates the influence of compaction on the de-coating efficiency and off-gas emissions, and its consequences for dross formation and recycling metal yield. Loose chips and two types of briquettes, one loosely compacted by uniaxial pressure and the other compacted by moderated-pressure-torsion to higher densities, were heated to 550 °C while analysing the off-gas emissions using FTIR. The briquettes were subsequently re-melted into a molten heel. Re-melting coated scrap multiplied the % wt of dross by a factor of 2 or 3, depending on the compaction pre-treatment, compared to re-melting uncoated aluminium. The densest briquettes emitted less than half the CO2 and CO gases during de-coating and formed significantly more dross. Compaction to the lower densities showed no tangible effects. The effect of de-coating compacted materials or not was small (± 2% wt dross), which was attributed to carbonaceous residues remaining after the thermal treatment. In conclusion, high compactions by torsion limit the de-coating reactions, which depend on factors such as temperature and gas transport. A complete removal of the organic residues is critical for a more sustainable recycling with less dross generated.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Avoid common mistakes on your manuscript.
Introduction
Efficient and sustainable recycling processes are critical to meet the increasing demand for aluminium while lowering production costs and environmental impacts. Recycling aluminium is less energy-intensive than primary production, which is one of the main reasons behind its associated lower emissions and environmental impacts [1]. The energy required to produce 1 tonne of secondary aluminium, based on European data, can vary between 2 and 9 GJ [2]. Damgaard [3] reported that recycling 1 tonne of aluminium-containing scrap and using the secondary metal produced to substitute primary aluminium saves between 5 and 19 tonne CO2 equivalent of global warming contributions. These variations in energy efficiency and emissions of the secondary route depend on the energy sources, the type and quality of the scrap and the consequent choice of pre-treatment, re-melting and refining processes.
In 2019, the International Aluminium Association registered a record global intake of post-consumer scrap for recycling of 20 million tonnes. The heaviest proportion, 5.3 million tonnes, was used packaging [4]. The main drivers of recycling packaging are their short lifetimes and the valuable wrought alloys they are made of. However, recycling thin sheet packaging materials can be complex due to their high surface-to-mass ratio and the presence of organic residues, coatings, and labels [5]. Thin shapes are more exposed to the atmosphere and oxidized during high-temperature processes, as demonstrated in [6, 7] for aluminium fabrication scrap and [8] for incineration bottom ash. Additionally, larger surface areas imply having more coatings, labels, and other surface-contaminants, which increase the aluminium losses and off-gas emissions during its re-melting. The aluminium losses are induced by reactions between the contaminants and the melt, forming oxides, sulphides (e.g., Al2S3) or carbides (e.g., Al4C3) [9].
Mixing oxidized or contaminated scrap with salts in rotary furnaces is a common practice, since the salt-flux protects the metal from oxidation and separates it from the non-metallic contaminants [10, 11]. However, using salts leads to salt slag residue, a toxic hazardous waste [12]. In contrast, salt-free re-melting processes require cleaner scrap and generate dross—a mix of oxides and entrapped Al—as residue, which is then recycled in rotary furnaces with salts, although alternative processes have been proposed [13]. Producing 1 tonne of secondary Al can generate up to 500 kg salt slag if re-melting in a rotary furnace with salt-fluxes or up to 80 kg dross if re-melting in a closed well furnace [2]. Decreasing the dross and salt slag residue quantities would be highly beneficial for the costs and environmental impacts of recycling. The presence of organic contamination on the scrap, as shown in [6, 14, 15], has a significant impact.
Preconditioning the scrap with a thermal treatment is an established method which makes it possible to re-melt initially dirty scrap without salts, described by e.g. in [16]. The thermal pre-treatment aims to remove as much moisture and organic contamination as possible without oxidizing the metal. According to the European Aluminium Scrap Standard EN 13920 coated packaging contains 71.5% metal, significantly less than the 86.1% of de-coated packaging [17], since most of the organics and volatiles (moisture, food residues, coatings and labels) are removed by the de-coating treatment. The thermal treatment also reduces the risks of health and safety hazards during re-melting, such as the generation of toxic, poisonous, or combustible gases (H2S, PH3, H2 and CH4) [9, 18]. Moreover, in salt-recycling processes, de-coating promotes the coalescence of the metal droplets, potentially reducing the amount of metal entrapped in the salt slag residue [19,20,21]. However, the volatile organic compounds (VOC’s) and other gases generated during the thermal de-coating pre-treatments can also pose health, safety and environmental risks and must be handled adequately, e.g. through afterburner and off-gas treatment, as discussed by Bateman [22]. The organic gases developed during de-coating or re-melting can also be utilised in several ways to improve the process energy efficiency [23].
A typical thermal de-coating of aluminium scrap can be divided into two phases. When the material is exposed to a sufficiently high temperature (above 250 °C), rapid decomposition of the organic molecules takes place (cracking). This releases volatile components such as short-chain hydrocarbons. The decomposition leaves a carbon-rich residue, which is gasified in the second stage, provided there is oxygen as a reaction partner in the gas atmosphere [24, 25]. To secure a complete removal of the organics during de-coating, the process parameters (temperature, atmosphere, duration) must be carefully controlled. Studies showed that treatments under inert gas-atmospheres and low temperatures can leave pyrolysis residues [26] which lead to re-melting losses by increasing the amount of dross generated in salt-free processes [25] or by lowering the coalescence of aluminium droplets and thus increasing the amount of aluminium entrapped in the salt slag residues when recycling in rotary furnaces [19]. On the other hand, using higher temperatures and O2-rich atmospheres increases the risk of oxidizing the scrap.
Pre-conditioning scrap by compaction into bales or briquettes can benefit recycling by facilitating transport, storage and charging operations. Research has also shown that compacting clean, dry aluminium swarf [27, 28] into briquettes increases its recycling efficiency in a salt-free process. Furthermore, briquetting thin aluminium foil can prevent high-temperature-oxidation and the consequent entrapment of small beads by the salt residues [29]. The benefits of compaction could be explained by, depending on the process, the reduction of the surface area exposed to oxidation, the higher density allowing the scrap to sink into the melt, or the enhanced metal–metal contact. However, for contaminated scrap, compacting may sometimes have negative consequences. A previous study showed that pressing coated aluminium chips into briquettes of low densities did not affect its recycling in salts. However, compacting to higher densities by the moderate-pressure torsion (MPT) method limited the de-coating efficiency and metal coalescence [21]. Steglich [25] re-melted used beverage cans (UBCs) without salt-flux and concluded that a thermal de-coating would reduce dross if conditions for stoichiometric thermolysis were applied for at least 30 min at 550–570 °C. The concept of stoichiometric thermolysis means that the exact amount of oxygen needed to react with the organics present is provided throughout the treatment. Steglich’s investigations argue that if conditions are not met, the char residues remaining when the total oxygen supply is too low (sub-stoichiometric conditions) or the oxidized aluminium due to too much oxygen (over-stoichiometric conditions) may lead to more dross and metal losses. One of the challenges of this approach is that it requires knowing the amount and composition of the organics present. In addition, the results suggested that the compaction state of the scrap also plays a role on the de-coating efficiency, being lower bale densities beneficial. Chamakos [30] thermally treated bundles of UBC bales and measured large differences between the temperature at the surface and centre of the bales as well as lower de-coating degrees inside, indicating that compaction may hinder de-coating by limiting heat transfer. Steglich and Chamakos investigated the recycling of UBCs, made of alloys with higher concentrations of Mg, which makes them more susceptible to oxidation, as demonstrated by Rossel [7].
The main contributions to knowledge in aluminium recycling by the current study is the detailed characterization of the de-coating off-gases during the thermal pre-treatment in different compaction states, and the consequences that the different thermal and compaction pre-treatment combinations can have on metal losses when re-melting without salts. The present study uses sheets of an alloy low in Mg, as it is the case for packaging materials as foils, laminated materials or flexible tubes, made of 8XXX and 1XXX alloys [31]. The stoichiometric thermolysis hypothesis proposed by Steglich [25] was used to test whether this estimate would give the optimal de-coating parameters.
Materials and Method
Materials
The materials were two coils of aluminium sheet AA8111 alloy with a thickness of 600 µm; one bare (uncoated) and one coated. The heel used in the re-melting experiments was of the same alloy. These sheets were fabricated to be used for roofing and they were chosen due to its low Mg content, which allows omitting the effect of Mg on oxidation metal losses during the de-coating and re-melting processes. The composition of the material’s production batches are provided in Table 2 in the Appendix. By using as-produced coated sheets instead of scrap, it was possible to isolate the influence that the coating and coating residues have for recycling from other contaminants, e.g., oil or food residues. The coating was dark grey and thicker (ca. 25 µm) on one side, light grey and thinner (5 µm) on the other. A preceding study [21] revealed that 75% wt of the coating (equal to 1.7 wt% of the sheet weight) was removable by a thermal treatment of 20 min at 550 °C under air. The residual 25% wt were oxides (BaSO4, TiO2 and SiO2) loosely adhered.
Shredding and Compaction Pre-treatment
The sheets were first shredded into chips, and then sieved with two square mesh sieves of 2 and 5 mm2. A subset of the chips was compacted into cylindrical briquettes of 4 cm diameter, each weighing 50 g, using a hydraulic press MTS 311. A subset was compacted by applying a uniaxial force of 100 kN for 5 s. Another subset was compacted by moderate-pressure-torsion (MPT), applying a compression load of 100 kN for 200 s while rotating the mould 4 times (speed of 1.2 rpm). The average density of the briquettes after compaction was 2.04 ± 0.04 g/cm3 for the uniaxial method and 2.22 ± 0.08 g/cm3 for the MPT method (just below the liquid Al density of 2.3 g/cm3). The height of the uniaxial briquettes was 1.96 ± 0.03 and of the MPT briquettes 1.80 ± 0.08 cm. Table 1 shows the experimental matrix of the materials and pre-treatment combinations.
Thermal De-coating Pre-treatment: Off-Gas Emissions
The samples were thermally treated in batches of 500 g inside a closed induction furnace connected to a Gasmet FTIR analyser. The heating rate was 350 °C/h and the temperature was held for 30 min at the top temperature of 550 °C. Since excess oxygen could lead to combustion and oxidation of the aluminium, the flow gas amount and composition were targeted to reach stoichiometric thermolysis conditions. The oxygen was calculated so that the hydrocarbon compounds contained in the organic matter would be converted to CO2 and CO in a ratio of 2:1. The gas mix with 5% O2 and 95% inert gas (N2) was set at a flow of 3 L/min after flushing the chamber with N2 at 180 °C. In the calculations it was assumed that the samples contained 2% wt organics and that the composition was 50% epoxy and 50% polyester, which would begin decomposing at 250 °C following Eqs. 1 and 2 respectively for 1 h and 22 min. These assumptions were based on the literature [32] and on a previous study [21] since the supplier did not provide information on the coating composition due to commercial considerations.
The top of the furnace chamber was water-cooled, and the outlet gas pipes had filters for particles larger than 1 µm, which were washed with ethanol between trials. The excess gas which was not analysed by the FTIR was connected to a scrubbing system and the residue collected in the outlet gas filters were analysed by gas chromatography and mass spectrometry (GS/MS).
Re-melting in Molten Heel: Dross Formation
The re-melting trials were conducted in an induction furnace operating at 2.5–3 kHz. The temperature was measured by a thermocouple placed inside the melt, and the melt was flushed with argon introduced to the hood at 10 L/min. First, the crucible with approximately 1 kg of aluminium heel was heated to 780 °C. Then the furnace was turned-off to skim the dross using a slotted spoon. The chips/briquettes (1 kg) were gradually charged into the melt at 750 °C using a small scoop. The power input to the induction furnace was adjusted to keep the temperature stable. Charging times ranged between 6 and 12 min. For the coated materials which had not been thermally pre-treated, the smoke arising from the combustion of the coating impeded the process, and the furnace power had to be turned off a few times to stir and sink the samples into the melt. After charging the melt was stirred again, and the temperature was raised to 780 °C before skimming the dross. Finally, the metal was cast at 750 °C. Once cold, the dross and the cast ingots were weighted to calculate the percentage of dross formation during re-melting (Eq. 3) and the metal yield (Eq. 4). For the first nine re-melting trials, the average weight of the dross skimmed-off the heel was 18.5 g ± 2.9 (1.73% ± 0.26 of its weight), and the Al casting residues remaining in the crucible weighed 15.0 ± 1.8 g. Both were considered negligible for the calculations.
Dross Re-melting in Salt-Flux: Dross Metal Content
The dross was re-melted in a resistance furnace. The salt-flux/dross ratio was 2:1 so that the dross would be completely submerged in the salt-flux, and the dross was sawed into pieces so that it would fit the crucible. First, the crucible filled with the mixed salts (68.6 wt% NaCl, 29.4 wt% KCl and 2 wt% CaF2, purchased separately and mixed before each trial) was placed inside the furnace at 800 °C. Once the salt was molten (after approximately 30 min) the dross was charged in two batches separated by 5 min. Manual stirring was applied during 5 s using a graphite stick, and the temperature was held for 10 min more after the second charge. The molten metal and salts were subsequently cast into a copper mould. The salts were washed away with water and the recovered metal weighed. The metal content of the dross was calculated using Eq. 5 and the total recycling yield with Eq. 6:
Dross Characterization Methods
Pieces of dross were polished and examined using Scanning Electron Microscope and Electron Probe Micro Analysis (SEM-EPMA). This allows distinguishing the phases and identifying some of the elements present. The equipment was a JEOL JXA-6500F Field Emission Electron Probe Microanalyzer. A small piece of the metal recovered from the dross re-melting, weighing approximately 1 g, was sent to ALS Scandinavia for ICP-MS trace element analysis.
Results and Discussion
Thermal Treatment
Figure 1 shows pictures of the chips and uniaxial briquettes before and after the thermal treatment. According to a prior study on the same material [21], a light brown/yellow colour is characteristic of the oxide residues remaining after a complete de-coating, while darker colours reveal the presence of carbonaceous residues.
For brevity in the discussion of the results, often when referring to the materials and briquettes tested, their names will be shortened to loose, uniaxial and MPT. The weight of the loose aluminium chips decreased after the thermal treatment by 1.51% ± 0.04, 1.43% for the uniaxial briquettes and 1.32% for the MPT briquettes. These values are the average and standard deviation of 2 trials for the chips, and the results of one trial for each of the briquette types. Based on the weight changes and the colour of the coating before and after the treatment, the uniaxially-compacted briquettes experienced a lower degree of de-coating than the loose chips, and the torsion-compacted briquettes (MPT) had the least degree of de-coating of the three compaction methods. This can be explained by the lower surface area and lower internal porosity of the MPT briquettes, limiting gas transport and the contact between oxygen and organics. A yellow/brown residue was condensed in the furnace lid and the filters of the outlet pipes. This was sent for GC/MS analysis at SINTEF Industry, identifying the components Phthalimide (C8H5NO2) and 1,2-Benzenedicarboxylic acid (C8H6O4) with a match factor of 99.4 and 99.5% respectively. The weight losses are smaller than those measured in the previous study using the same material, which were 1.7% wt for chips and uniaxial briquettes and 1.5% wt for MPT briquettes [33]. However, those trials were conducted in a muffle furnace in air, and the present trials in a thermolysis furnace with a lower oxygen concentration (5%). In addition, the amount of material treated per trial in this study is significantly higher than the previous study: 500 g vs. 60 g. To check the weight losses expected after a complete de-coating that does not leave dark carbonaceous residues, two more trials were conducted on chips using a different set-up. For each trial a tray with approximately 1 kg of chips was introduced in a muffle furnace at 550 °C. After 30 min, there were still dark residues in the chips, so the holding time was extended to 1 h. The average weight loss measured was 1.59 ± 0.02%, only slightly higher than measured from the chips treated in the thermolysis furnace. Thus, de-coating coated sheet industrially could lead up to 16 kg of weight losses per tonne of scrap treated. Assuming that all those volatiles are 50/50% wt epoxy/polyester, and the decomposition follows the thermolysis reactions described in Eqs. 1 and 2, fully de-coating 1 tonne of this coated chips would generate approximately 24 kg of CO2 and 8 kg of CO gases.
Although the time, temperature, gas flow and oxygen concentrations needed for the stoichiometric reactions to volatilize all organics had been calculated, dark residues remained for all trials. This suggests that the oxygen supplied, time or temperature were not sufficient to react with all the organics present. Alternatively, it could point to the unsuitable placement of the gas pipes. Both the inflow and outflow piping were connected through the furnace lid, while the materials were placed below, stacked inside a crucible. Future investigations should ensure optimal transport of gases with a moving material bed, e.g., use a rotary kiln while still ensuring briquette integrity.
De-coating Off-Gas Analysis
Figure 2 shows the off-gases generated during the thermal pre-treatment for all compaction types.
For clarity, the hydrocarbons are grouped according to their chemical structure as aliphatic and aromatic hydrocarbons. Aliphatic hydrocarbons include methane, ethane, propane, heptane, ethene and propene. The aromatic hydrocarbons include benzol, styrene and phenol. Additionally, two aldehydes, formaldehyde and acetaldehyde, were measured.
The results reveal that the CO2 concentration decreases significantly with increased compaction. The measured CO2 peaked at 42,506 ppm for loose chips, 35,340 ppm for uniaxial and 24,000 ppm for MPT. The same trend applies for CO (Loose: 9448 ppm, Uniaxial: 7995 ppm, MPT: 5800 ppm), aliphatic hydrocarbons (Loose: 3660 ppm, Uniaxial: 3118 ppm, MPT: 1722 ppm) and aromatic hydrocarbons (Loose: 2644 ppm, Uniaxial: 2146 ppm, MPT: 1630 ppm). The gas evolution started at temperatures between 175 and 190 °C. The emission of CO2, CO and hydrocarbons peaked after 1 h at around 320 °C for MPT and 400 °C for chips and uniaxial briquettes. The first phase, scission, and decomposition of organics, lasted until 500–515 °C. After this temperature was reached, no hydrocarbons were present in the off-gas and the second phase began: the gasification of residual carbon, releasing CO2 and CO at a much slower rate. These two phases (although the off-gases were previously not analysed in such detail) correspond with those described in literature for organic coatings on aluminium [24] and magnesium [34]. The de-coating gas evolution has also been monitored by Al Mahmood [35] and Gökelma [20] when treating polymer-laminated thin aluminium (coffee capsules), although in those cases the furnace atmosphere was inert.
To correlate the findings with the theoretical concept of stochiometric thermolysis, the total gas volumes were calculated from the FTIR measurements and averaged for each pre-treatment route. The calculation of released gases refers to the time period where the sample is above 175 °C, starting from the initial heating phase where the first volatile components are measured by the FTIR and then until cooling down below 175 °C. This temperature range/profile differs from that reported for the decomposition of other coatings (commencing at 250 °C) reported in literature. This could be due to temperature differences between the samples and the thermocouple. To correctly calculate the total amount of gas, the time interval in which the gases were measured by FTIR was chosen. Accordingly, the total generation of gaseous components was calculated by Eq. 7:
\({V}_{i,total}:\) Total volume of generated gaseous component i [L].
\({t}_{0}:\) point in time where the sample reaches 175 °C [min] \({t}_{1}:\) point in time where the sample falls below 175 °C [min] \({c}_{i}:\) concentration of gaseous component i at specific time [%] \(\dot{V}:\) gas flow [L/min].
The results are summarized in Fig. 3. The total volume of gas generated decreased with compaction. This applies to CO2 and CO as well as to organic volatile compounds. Treating 500 g of loose chips generated 3.44 litres (± 0.978 L) while the uniaxial briquettes released 2.77 L (± 1.33 L) and the MPT briquettes only produced 1.2 L (± 1.47 L). Hydrocarbons (aliphatic and aromatic) volumes were similar when treating loose or uniaxial briquettes but lower for the MPT briquettes. Aldehydes were predominantly released during the thermal pre-treatment of loose chips with up to 0.143 L (± 0.08 L). However, the estimated variability is larger than the differences between compaction states. This may be because part of the briquettes fell apart during handling, as shown in the briquettes cross-sections of Fig. 3b.
The ratio between the average total CO2 and CO released was higher (4.2) for chips and uniaxial briquettes than for the MPT briquettes (3.7). Higher values indicate a higher degree of combustion during the de-coating reactions. The decreasing off-gas generation, particularly CO2 and CO, as well as the decreasing CO2/CO ratio for the denser briquettes (MPT), supports the hypothesis raised in the previous section: oxygen availability is crucial for organics removal, and therefore compacting coated materials into briquettes of lower surface areas limits the efficiency of the de-coating. The internal porosity of the briquettes was measured in a previous study [33] as 15% for uniaxial and 5% for MPT. Therefore, gas transport was very limited inside the MPT. Chamakos [30] attributed the incomplete de-coating of UBC bales to their low thermal conductivity, since the temperatures measured in the center of the bales were below those required for de-coating. But due to the small size of the briquettes in this study, it is assumed that the temperature differences between the surface and the centre are negligible and that insufficient gas transport is the main factor behind the influence of compaction on de-coating.
The ideal gas law was used to convert the volumes into mass, considering that the temperature of the off-gases was 180 °C during measurement. De-coating 1 tonne of the materials under the present operation conditions would generate 8.1 kg CO2 eq. per tonne scrap for the chips, 6.6 kg CO2 eq. for the briquettes and only 2.8 kg CO2 eq. for the MPT briquettes. Thus, high densifications reduce the gases from re-melting/pre-treatment. This could be beneficial from the point of view that it reduces the amount of off-gases which need to be further treated and the process direct emissions, although on the other side the off-gases could be reused internally to save energy, as discussed in [23]. The next section investigates the implications for the performance of the re-melting process.
Re-melting
The coated samples behaved very differently depending on whether they had been thermally treated or not. If untreated, they burned as soon as they were charged to the furnace, while those thermally pre-treated did not. When comparing the untreated samples in various compaction states, the most compacted briquettes (MPT) produced less smoke and flames. Thus, in industrial processes which re-melt dirty scrap without pre-treatment (e.g., in rotary furnaces with salts), compacting the bales to higher densities could be beneficial for process control and safety.
Figure 4 shows the dross generation relative to the initial weight of the scrap (Eq. 3) and the average metal yield (Eq. 4) for each pre-treatment route. Re-melting the uncoated, bare chips generated the least amount of dross (9% wt). Even if the weight of the coating was less than 2% of the scrap charged, the presence of the coating led to at least twice as much dross generation for all cases: values around 20–30 wt% of the scrap charged. The differences between the thermally treated and untreated coated samples were ~ 2% wt. This difference was lower than expected, as the removal of organic contaminants and the prevention of the combustion reactions during re-melting were thought to have a larger impact. However, as discussed, the thermal treatment only removed part of the organics. Regarding the effect of the degree of compaction, re-melting chips and uniaxial briquettes generated similar amounts of dross relative to the weight of scrap charged (~ 19–22%), while the MPT samples generated higher relative amounts of dross (28–30% wt). The metal yield results follow the inverse trend; higher metal yield (94% wt) for the uncoated material, lower for the chips and uniaxial briquettes (87–89% wt), lowest for the MPT briquettes (83–84% wt), and ~ 2% wt differences due to thermal treatment. The de-coating weight losses presented in the previous section were used to estimate the initial metal content of the samples before re-melting and the metal recovery: ratio of metal recovered with respect to the metal charged into the furnace. The average numbers for metal recovery slightly reduced the differences between the thermal pre-treatment route and directly melting. All the re-melting data is provided in the online supplementary material.
a Dross related to wt scrap. b % wt Metal Yield from cast ingots. c Dross metal content d Total recycling yield. Each bar represents the average and STD for each conditioning route: compaction (loose chips/uniaxial briquettes/MPT briquettes) and thermal treatment (coated/de-coated). Chips uncoated is untreated bare Al
The presence of organic residues seems to be the major factor behind dross formation, irrespective of pre-treatment of the chips and briquettes or by just adding to the aluminium melt. The results show that the combustion of the coating during melting of the chips or briquettes produced the same amount of dross as the de-coated chips and briquettes. The coating residues may consist of decomposed but not removed products of thermal pre-treatment, such as carbon and non-decomposed long chain hydrocarbons (CxHyOz). These will likely react with the liquid aluminium during re-melting and cause dross formation and metal losses due to the formation of aluminium carbide and oxide (see Eqs. 8 & 9). Additionally, re-oxidation of aluminium with CO2 and CO can occur (see Eqs. 10 & 11). This has also been reported in previous studies [36].
The metal content of the dross, obtained by re-melting it in salt-flux, is represented in Fig. 4c). The results confirm the expected high metallic content of the dross from re-melting uncoated Al: 97.7 ± 0.3%. The dross from the coated and the thermally treated chips gave similar metal yields (92.2 ± 1.6% and 91.8 ± 0.7% respectively) to the coated uniaxial briquettes (92.0 ± 1.2%). The dross from thermally treated uniaxial briquettes, MPT untreated and the MPT treated briquettes contained less metal (90.3 ± 0.3%, 89.9 ± 1.1% and 90.4 ± 0.3% respectively).
Finally, the total recycling yield in Fig. 4d) shows that, if the aluminium from the dross is recovered, recycling coated aluminium leads to slightly higher overall losses than bare aluminium, which increase if the scrap is compacted to high densities by torsion. Although these differences may seem small, at an industrial scale they would have a significant environmental and economic impact. Due to the higher energy-intensity and environmental impacts associated with primary aluminium production compared to recycling [1], the optimization of the recycling processes is key for a more sustainable Al production. For the studied process, high densification decreased the metal yield by 4–5%. Assuming the aluminium would be recovered from the dross by a salt-flux process as efficient as these re-melting trials at laboratory scale, the increase in total metal yield when melting loose chips instead of highly compacted MPT briquettes corresponds to 10.5 kg per tonne of treated material. With a CO2 equivalent of 22.4 kg/kg of primary aluminium [37] not recovering that aluminium would correspond to loosing potential CO2 savings of 235.2 kg/tonne of treated scrap. As the degree of compaction of the scrap increases, the total metal yield decreases, raising the carbon footprint of the re-melting process. Figure 5 relates the total metal yield for the compaction routes to the potential CO2 savings which could be achieved if the Metal Yield of the process would be improved to 100%.
Furthermore, to recover the metal from the dross implies additional transport, energy, and resources to treat it in a rotary furnace and generates salt-slag residues that need to be processed as well [2]. Thus, minimising the amount of organic contamination introduced into the melt by applying a successful thermal pre-treatment in loose/loosely compacted scrap would reduce the metal lost to the dross, bringing large environmental and economic savings.
Dross Characterization
The SEM/EPMA analysis of the dross samples is displayed in Fig. 6. The square in the top left of each bundle of images shows the secondary electron image and the rest are qualitative colour maps of the concentration of specific elements. Approximate concentrations can be deduced using the colour scales.
All dross samples contained mostly aluminium. The dross from the uncoated material (Fig. 6a) contains magnesium and calcium oxides, as well as silicon and iron phases expected in the alloy. In contrast, the dross from the coated samples (both thermally treated (Fig. 6b) and untreated) contained titanium, silicon, barium, sulphur, and calcium. Most of these elements were arranged into layers and agglomerates. This agrees with previous analysis [21] which identified BaSO4, TiO2, and SiO2 as residues in the coating which are not removable by thermal treatment, and remain loosely adhered to the surface. Meskers [38] observed TiO2, CaO, BaSO4 and SiO2 residues as well on magnesium scrap. They are added as pigments or fillers. Since the samples were embedded in epoxy it was not possible to identify whether carbon-containing coating residues were also present. There were no significant compositional differences between the dross from re-melting de-coated samples or from directly charging them into the melt. The elemental composition of the metal recovered from the dross is displayed in Table 3 in the Appendix.
The results suggest that the salt-flux separates the coating oxide residues from the metal as there is no significant increase of neither titanium, barium nor silicon when comparing the results to the initial alloy composition (Table 1). There was an increase in sodium, likely from the NaCl present in the salt-flux, and a decrease in Mg for the uncoated material. Magnesium could have been evaporated or oxidized during the first re-melting or removed by reaction with the salt-flux. This is consistent with published experimental [21] and thermodynamic [39] recycling studies for aluminium and magnesium [34].
Since scrap compaction brings logistic and operational benefits, the authors propose the following practice for recycling packaging scrap in a more environmentally friendly way, minimizing dross:
-
1.
Transport the scrap compacted into bales to the recycling facility.
-
2.
Loosen up or shred the bales for sorting and thermal pre-treatment with controlled air flow and off-gas treatment.
-
3.
Once the organics and volatiles are fully removed, consider compacting the scrap into briquettes to ease its charging and sinking (not necessary for furnaces with vortex). High densification is not needed.
-
4.
Recover Al from dross by salt-flux recycling or other suitable treatments/uses.
Conclusions
This study investigated the effects of compacting coated aluminium chips of an alloy low in Mg on the efficiency of the thermal de-coating and re-melting processes by comparing dross generation, de-coating off-gases and overall metal yield. The following conclusions were drawn:
Thermal De-coating Pre-treatment
-
Oxygen availability is crucial for maximising the gasification of carbon from coatings.
-
A thermal pre-treatment of 30 min at 550 °C under a 5% O2–95% N2 atmosphere, only partly removed the organic components of the coating, possibly due to a low O2 residence time inside the furnace. Further work should investigate the optimal transport of gases, e.g., in a rotary kiln with a moving material bed.
-
Compaction into briquettes of high densities by torsion lowers the de-coating efficiency due to limiting the gas transport inside the briquette, due to the briquette’s lower surface area and internal porosity.
-
The main gaseous products were CO2 and CO, and their emission decreased for higher compactions, as well as the CO2/CO ratio decreasing. The loose chips released 3.44 L of VOCs, uniaxially compacted briquettes 2.77 L and the densest briquettes (MPT) 1.2 L.
-
Other gas emissions included aliphatic hydrocarbons (methane, ethane, heptane, ethene and propene), aromatic (benzol, styrene and phenol) hydrocarbons and aldehydes (formaldehyde and acetaldehyde).
Re-melting
-
Applying a thermal de-coating pre-treatment prevents flames and smoke during re-melting, making the process safer and more stable.
-
The organic residues from the coating after pre-treatment were the main factor increasing dross and lowering recycling yield. The incomplete de-coating did not reduce the dross generated or improve the recycling yield significantly compared to re-melting without de-coating, despite avoiding combustion gases above the molten aluminium.
-
The briquettes compacted to higher densities by torsion (MPT) produced the highest amount of dross (29% wt of the charged samples) and the lowest total recycling yield (97% wt), due to the compaction limiting the burn-off of the organic residues. This has economic and sustainability implications at the industrial scale.
-
Melting loose material results in the highest total metal yields. This potentially saves environmental impacts, as it reduces the demand for primary aluminium. In this context, the melting of loose material provides an estimated reduction of 235 kg CO2 eq/tonne of treated scrap compared to the highly densified (MPT) briquettes.
-
Removing the organics from aluminium scrap before re-melting helps minimising the production of dross, hereby reducing efforts for an additional re-melting process in salt-flux. Reducing the amount of dross accounts for a decrease in total energy, resources and salt-slag residues associated with the recycling process.
References
Olivieri G, Romani A, Neri P (2006) Environmental and economic analysis of aluminium recycling through life cycle assessment. Int J Sust Dev World 13(4):269–276. https://doi.org/10.1080/13504500609469678
Cusano G, Rodrigo Gonzalo M, Farrell F, Remus R, Roudier S, Delgado Sancho L (2017) Best available techniques (bat) reference document for the non-ferrous metals industries. Ind Emiss Dir. https://doi.org/10.2760/8224
Damgaard A, Larsen AW, Christensen TH (2009) Recycling of metals: accounting of greenhouse gases and global warming contributions. Waste Manage Res 27(8):773–780. https://doi.org/10.1177/0734242X09346838
IAI (2021) Material flow model. URL: https://international-aluminium.org/resource/iai-material-flow-model-2021-update/
Tabereaux AT, Peterson RD (2014) Ch. 2.5 Aluminum production. In: Seetharaman S (ed) Treatise on process metallurgy. Elsevier, Boston, pp 839–917
Xiao Y, Reuter MA (2002) Recycling of distributed aluminium turning scrap. Miner Eng 15(11):963–970. https://doi.org/10.1016/S0892-6875(02)00137-1
Rossel H (1990) Fundamental investigations about metal loss during remelting of extrusion and rolling fabrication scrap. In: Bickert CM (ed) Light metals 1990. Springer, Cham
Gökelma M, Vallejo-Olivares A, Tranell G (2021) Characteristic properties and recyclability of the aluminium fraction of mswi bottom ash. Waste Manage 130:65–73. https://doi.org/10.1016/j.wasman.2021.05.012
van Schaik A, Reuter MA (2014) Ch. 22 Material-centric (aluminium and copper) and product-centric (cars, weee, tv, lamps, batteries, catalysts) recycling and dfr rules. In: Worrell E, Reuter MA (eds) Handbook of recycling. Elsevier, Amsterdam
Milani V, Timelli G (2023) Solid salt fluxes for molten aluminum processing—a review. Metals. https://doi.org/10.3390/met13050832
Schmitz C (2014) Handbook of aluminium recycling: mechanical preparation, metallurgical processing, heat treatment. Vulkan-Verlag, Essen
Tsakiridis PE, Oustadakis P, Agatzini-Leonardou S (2013) Aluminium recovery during black dross hydrothermal treatment. J Environ Chem Eng 1(1):23–32. https://doi.org/10.1016/j.jece.2013.03.004
Ünlü N, Drouet MG (2002) Comparison of salt-free aluminum dross treatment processes. Resour Conserv Recycl 36(1):61–72. https://doi.org/10.1016/S0921-3449(02)00010-1
Xiao Y, Reuter MA, Boin U (2005) Aluminium recycling and environmental issues of salt slag treatment. J Environ Sci Health 40(10):1861–1875. https://doi.org/10.1080/10934520500183824
Bao S, Kvithyld A, Bjørlykke GA, Sandaunet K (2023) Recycling of aluminum from aluminum food tubes. In: Broek S (ed) Light metals 2023. Springer, Cham, pp 960–966
Capuzzi S, Timelli G (2018) Preparation and melting of scrap in aluminium recycling: a review. Metals 8:249. https://doi.org/10.3390/met8040249
Boin UMJ, Bertram M (2005) Melting standardized aluminum scrap: a mass balance model for Europe. JOM 57(8):26–33. https://doi.org/10.1007/s11837-005-0164-4
Stark TD, Martin JW, Gerbasi GT, Thalhamer T, Gortner RE (2012) Aluminum waste reaction indicators in a municipal solid waste landfill. J Geotech Geoenviron Eng 138(3):252–261. https://doi.org/10.1061/(ASCE)GT.1943-5606.0000581
Capuzzi S, Kvithyld A, Timelli G, Nordmark A, Engh TA (2017) Influence of coating and de-coating on the coalescence of aluminium drops in salt. In: Ratvik AP (ed) Light metals 2017. Springer, Cham, pp 1115–1121
Gökelma M, Diaz F, Öner IE, Friedrich B, Tranell G (2020) An assessment of recyclability of used aluminium coffee capsules. In: Tomsett A (ed) Light metals 2020. Springer, Cham, pp 1101–1109
Vallejo-Olivares A, Høgåsen S, Kvithyld A, Tranell G (2022) Thermal de-coating pre-treatment for loose or compacted aluminum scrap and consequences for salt-flux recycling. J Sustain Metall 8(4):1485–1497. https://doi.org/10.1007/s40831-022-00612-x
Bateman W, Guest G, Evans R (1999) Decoating of aluminium products and the environment. In: Eckert CE (ed) Light metals. Springer, Cham, pp 1099–1105
Haraldsson J, Johansson MT (2018) Review of measures for improved energy efficiency in production-related processes in the aluminium industry—from electrolysis to recycling. Renew Sustain Energy Rev 93:525–548. https://doi.org/10.1016/j.rser.2018.05.043
Kvithyld A, Meskers CEM, Gaal S, Reuter M, Engh TA (2008) Recycling light metals: optimal thermal de-coating. JOM 60(8):47–51. https://doi.org/10.1007/s11837-008-0107-y
Steglich J, Friedrich B, Rosefort M (2020) Dross formation in aluminum melts during the charging of beverage can scrap bales with different densities using various thermal pretreatments. JOM 72(10):3383–3392. https://doi.org/10.1007/s11837-020-04268-4
Steglich J, Dittrich R, Rombach G, Rosefort M, Friedrich B, Pichat A (2017) Dross formation mechanisms of thermally pre-treated used beverage can scrap bales with different density. In: Ratvik AP (ed) Light metals 2017. Springer, Cham, pp 1105–1113
Puga H, Barbosa J, Soares D, Silva F, Ribeiro S (2009) Recycling of aluminium swarf by direct incorporation in aluminium melts. J Mater Process Technol 209(11):5195–5203. https://doi.org/10.1016/j.jmatprotec.2009.03.007
Puga H, Barbosa J, Ribeiro CS (2013) Factors affecting the metal recovery yield during induction melting of aluminium swarf. Mater Sci Forum 730–732:781–786
Vallejo-Olivares A, Philipson H, Gökelma M, Roven HJ, Furu T, Kvithyld A et al (2021) Compaction of aluminium foil and its effect on oxidation and recycling yield. In: Perander L (ed) Light metals 2021. Springer, Cham, pp 735–741
Chamakos N, Koklioti M, Tzevelekou T, Fiampouri A, Contopoulos I, Anestis A et al (2023) Towards the efficient recycling of used beverage cans: numerical study and experimental validation. In: Broek S (ed) Light metals 2023. Springer, Cham, pp 942–948
Piergiovanni L, Limbo S (2016) Food packaging materials. In: Salvatore Parisi RP (ed) Chemistry of foods Springer briefs in molecular science, 1st edn. Springer, Cham
Steglich J (2020) Krätzebildung in aluminiumschmelzen durch rückstände der thermischen vorbehandlung von getränkedosenschrotten. Schriftenreihe des IME, Aachen
Vallejo-Olivares A, Høgåsen S, Kvithyld A, Tranell G (2022) Effect of compaction and thermal de-coating pre-treatments on the recyclability of coated and uncoated aluminium. In: Eskin D (ed) Light metals 2022. Springer, Cham, pp 1029–1037
Meskers CEM, Reuter MA, Boin U, Kvithyld A (2008) A fundamental metric for metal recycling applied to coated magnesium. Metall Mater Trans B 39(3):500–517. https://doi.org/10.1007/s11663-008-9144-8
Al Mahmood A, Hossain R, Sahajwalla V (2020) Investigation of the effect of laminated polymers in the metallic packaging materials on the recycling of aluminum by thermal disengagement technology (tdt). J Clean Prod 274:122541. https://doi.org/10.1016/j.jclepro.2020.122541
Dittrich R, Friedrich B, Rombach G, Steglich J, Pichat A (2017) Understanding of interactions between pyrolysis gases and liquid aluminum and their impact on dross formation. In: Ratvik AP (ed) Light metals 2017. Springer, Cham, pp 1457–1464
Norgate TE, Jahanshahi S, Rankin WJ (2007) Assessing the environmental impact of metal production processes. J Clean Prod 15(8):838–848. https://doi.org/10.1016/j.jclepro.2006.06.018
Meskers CEM, Xiao Y, Boom R, Boin U, Reuter MA (2007) Evaluation of the recycling of coated magnesium using exergy analysis. Miner Eng 20(9):913–925. https://doi.org/10.1016/j.mineng.2007.02.006
Hiraki T, Miki T, Nakajima K, Matsubae K, Nakamura S, Nagasaka T (2014) Thermodynamic analysis for the refining ability of salt flux for aluminum recycling. Materials 7(8):5543–5553. https://doi.org/10.3390/ma7085543
Acknowledgements
The authors would like to gratefully acknowledge the Research Council of Norway for funding the projects Extreme-Alloys and Coatings for Space and other Extreme Applications (NFR Project nr. 310048) and Alpakka—Circular Aluminium Packaging in Norway (NFR Project nr. 296276), to Speira Holmestrand for the materials and to the departments of Process Metallurgy and Metal Recycling (IME) at RWTH Aachen University, and Materials Science and Engineering (IMA) at NTNU for the experimental equipment and support. The authors acknowledge Anders Brunsvik (SINTEF) for GC/MC analysis, Morten Peder Raanes (NTNU) for SEM-EPMA analysis and Anne Kvithyld (SINTEF) for the valuable discussions.
Funding
Open access funding provided by NTNU Norwegian University of Science and Technology (incl St. Olavs Hospital - Trondheim University Hospital).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
The contributing editor for this article was Markus Reuter.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Appendix
Appendix
The composition of the coated material was calculated from 9 samples, 4 of which had been thermally treated before the molten heel re-melting, and 5 from samples without thermal treatment. The uncoated material composition was calculated from 2 samples. There were no evident differences attributable to the application of different compaction or thermal pre-treatment routes. Therefore, the averages were calculated based on the initial aluminium sheet composition used for the samples (coated or uncoated).
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Vallejo-Olivares, A., Gertjegerdes, T., Høgåsen, S. et al. Effects of Compaction and Thermal Pre-treatments on Generation of Dross and Off-Gases in Aluminium Recycling. J. Sustain. Metall. 10, 69–82 (2024). https://doi.org/10.1007/s40831-023-00773-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40831-023-00773-3