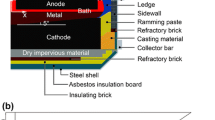
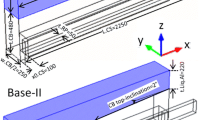
Abstract
A finite element model was developed to determine the impact of cathode material and shape on current density in an aluminum electrolysis cell. For the cathode material, results show that increased electrical resistivity leads to a higher cathode voltage drop; however, the horizontal current is reduced in the metal. The horizontal current magnitude for six different cathode materials in decreasing order is graphitized, semi-graphitized, full graphitic, 50% anthracite (50% artificial graphite), 70% anthracite (30% artificial graphite), 100% anthracite. The modified cathode shapes with an inclined cathode surface, higher collector bar and cylindrical protrusions are intended to improve horizontal current and flow resistance. Compared to a traditional cathode, modified collector bar sizes of 70 mm × 230 mm and 80 mm × 270 mm can reduce horizontal current density component Jx by 10% and 19%, respectively, due to better conductivity of the steel. The horizontal current in the metal decreases with increase of cathode inclination. The peak value of Jx can be approximately reduced by 20% for a 2° change in inclination. Cylindrical protrusions lead to local horizontal current increase on their tops, but the average current is less affected and the molten metal is effectively slowed down.











Similar content being viewed by others
References
N. Kandev and H. Fortin, Light Metals, ed. G. Bearne (Warrendale: TMS, 2009), pp. 1061–1066.
O. Zikanov, A. Thess, P.A. Davidson, and D.P. Ziegler, Metall. Trans. B 31, 1541 (2000).
S. Das, Y. Morsi, and G. Brooks, JOM 66, 235 (2014).
S. Das, G. Brooks, and Y. Morsi, Metall. Trans. B 42, 243 (2011).
M. Li, J. Cent. South Univ. (Sci. Technol.) 40, 562 (2009).
H. Sun, O. Zikanov, and D.P. Ziegler, Fluid Dyn. Res. 35, 255 (2004).
V. Bojarevics and K. Pericleous, Light Metals, ed. G. Bearne (Warrendale: TMS, 2009), pp. 569–574.
B. Li, Light Metals, ed. S.J. Lindsay (Warrendale: TMS, 2011), pp. 1029–1033.
B. Li, Light Metals, ed. C.E. Suarez (Warrendale: TMS, 2012), pp. 865–868.
S. Das and G. Littlefair, Light Metals, ed. S.J. Lindsay (Warrendale: TMS, 2011), pp. 847–851.
R. Kaenel and J. Antille, Light Metals, ed. S.J. Lindsay (Warrendale: TMS, 2011), pp. 569–574.
M. Dupuis and R.D. Peterson, Light Metals, ed. R.D. Perterson (Warrendale: TMS, 2000), pp. 169–178.
J. Dreyfus, L. Rivoaland, and S. Lacroix, Light Metals, ed. A.T. Tabereaux (Warrendale: TMS, 2004), pp. 603–608.
M. Blais, M. Desilets, and M. Lacroix, Appl. Therm. Eng. 58, 439 (2013).
S. Das, G. Brooks, and Y. Morsi, Metall. Trans. B 42, 243 (2011).
J. Zoric, J. Thonstad, and T. Haarberg, Metall. Trans. B 30, 341 (1999).
H. Fortin, N. Kandev, and M. Fafard, Finite Elem. Anal. Des. 52, 71 (2012).
N.X. Feng, J.P. Peng, Y.W. Wang, Y.Z. Di, and X.A. Liao, Light Metals, ed. B. Sadler (Warrendale: TMS, 2012), pp. 549–552.
N. Feng, China Patent, CN 102400176A, 2012/04/04.
N. Feng, Aluminum Electrolysis, 188 (2006).
P. Reny and S. Wilkening, Light Metals, ed. D.P. Peterson (Warrendale: TMS, 2000), pp. 1005–1010.
H.A. Øye and B.J. Welch, JOM 50, 18 (1998).
D. Lombard, T. Beheregaray, B. Feve, and J.M. Jolas, Light Metals, ed. B.J. Welch (Warrendale: TMS, 1998), pp. 653–658.
Acknowledgements
The authors are grateful for the financial support by the National Nature Science Foundation of China (Grant Nos. 51204044 and 51434005) and the National Key Technology R&D Program of China (No. 2015BAB04B03).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Song, Y., Peng, J., Di, Y. et al. The Impact of Cathode Material and Shape on Current Density in an Aluminum Electrolysis Cell. JOM 68, 593–599 (2016). https://doi.org/10.1007/s11837-015-1719-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11837-015-1719-7