Abstract
Several Barbarea spp. (Brassicaceae) have been tested as trap crops for the diamondback moth Plutella xylostella L. (Lepidoptera: Plutellidae). The use of trap crops can be affected by their susceptibility to other pests, especially if the purpose is to reduce insecticide use. Barbarea rupicola Moris, B. verna (Mill.) Asch., and B. vulgaris Aiton (types G and P) (Brassicaceae) were tested for their susceptibility to the cabbage whitefly Aleyrodes proletella L. (Hemiptera: Aleyrodidae). The percentage of plants showing infestation by cabbage whiteflies ranged from 50% in G-type B. vulgaris and 8.3% in B. verna to no infestation at all in B. rupicola and P-type B. vulgaris. On the other hand, 95.8% of P-type plants showed symptoms of powdery mildew, Erysiphe cruciferarum Opiz ex L. Junell (Erysiphales: Erysiphaceae), while the G type and the other Barbarea spp. were unaffected by this pathogen. Additionally, the G and P types were used in two-choice oviposition preference tests to compare their attractiveness to the small white butterfly Pieris rapae L. (Lepidoptera: Pieridae). No significant differences in total oviposition per plant were found between the two types, but within-plant differences show that the small white butterfly prefers to oviposit on the adaxial leaf side in the P type. This study indicates that in locations where the cabbage whitefly is an economic pest, B. verna, which can also be used as a dead-end trap crop for the diamondback moth, could be chosen over G-type B. vulgaris.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Wintercress, Barbarea vulgaris Aiton (Brassicaceae), and other Barbarea spp. have been tested as traps crops and insectary plants for the management of diamondback moth, Plutella xylostella L. (Lepidoptera: Plutellidae) (Idris and Grafius 1994, 1995; Shelton and Nault 2004; Badenes-Pérez et al. 2005, 2014, 2017; Shelton and Badenes-Pérez 2006; Badenes-Pérez 2019). Barbarea vulgaris has two morphologically distinct forms, which have glabrous (G type) and pubescent (P type) leaves, respectively, and which also differ in their glucosinolate and saponin content, number of leaves, leaf size, and habitat adaptation (Agerbirk et al. 2003a, 2015; Kuzina et al. 2009; Nielsen et al. 2010b; Hauser et al. 2012, 2021; Badenes-Pérez et al. 2014; Christensen et al. 2014, 2016; Heimes et al. 2016; Badenes-Pérez and López-Pérez 2018). G-type B. vulgaris (henceforth the G type) contains the saponins 3-O-β-cellobiosylhederagenin and 3–0-β-cellobiosyloleanolic acid, which act as feeding deterrents for diamondback moth larvae, while P-type B. vulgaris (henceforth the P type) does not contain these saponins and is susceptible to the diamondback moth (Agerbirk et al. 2003a; Badenes-Pérez et al. 2014). Barbarea verna (Mill.) Asch. also contains sufficient amounts of these triterpenoid saponins to make this plant resistant to the diamondback moth (Badenes-Pérez et al. 2014). Because of this resistance to the diamondback moth, the G type and B. verna have been proposed as dead-end trap crops for this insect pest (Badenes-Pérez et al. 2004, 2005, 2014; Lu et al. 2004; Shelton and Nault 2004). Barbarea rupicola Moris contains low amounts of these saponins and some diamondback moth larvae can survive on it (Badenes-Pérez et al. 2014). On the other hand, these Barbarea spp., including the P type, are highly attractive to ovipositing diamondback moth and have, thus, potential to be used as trap crops, though only the G type and B. verna can be used as dead-end trap crops for this insect (Badenes-Pérez et al. 2014).
Besides the differences in resistance to the diamondback moth, the G and P types show different levels of resistance to other herbivores. The G type has shown resistance to the flea beetle Phyllotreta nemorum L. (Coleoptera: Chrysomelidae) (Agerbirk et al. 2001; Nielsen et al. 2010a; Kuzina et al. 2011; Hauser et al. 2021), to the western flower thrips Frankliniella occidentalis Pergande (Thysanoptera: Thripidae), and to the ascomycete fungus that causes powdery mildew on Brassicaceae Erysiphe cruciferarum Opiz ex L. Junell (Erysiphales: Erysiphaceae) (henceforth powdery mildew) (Badenes-Pérez and López-Pérez 2018). Compared to the P type, the G type is a worse host to the root-knot nematode Meloidogyne incognita (Kofoid & White) Chitwoodi (Tylenchida: Heteroderidae) (Badenes-Pérez and López-Pérez 2018). The G type is also less susceptible to the specialist turnip sawfly Athalia rosae L (Hymenoptera: Tenthredinidae) and to some species of generalist molluscs than the P type, but both types are equally attractive to the generalist cabbage moth Mamestra brassicae L. (Lepidoptera: Noctuidae) (Christensen et al. 2018). In contrast, the P type shows resistance to the oomycete pathogen that causes white rust Albugo candida (Pers.) Kuntze (Peronosporales: Albuginaceae), while the G type is susceptible to it (van Mölken et al. 2014; Christensen et al. 2014; Heimes et al. 2014; Hauser et al. 2021).
The cabbage whitefly Aleyrodes proletella L. (Hemiptera: Aleyrodidae) (henceforth cabbage whitefly) and the small white, also known as cabbage white butterfly and imported cabbageworm, Pieris rapae L. (Lepidoptera: Pieridae) (henceforth small white), can be economic pests in cruciferous crops (Shelton et al. 1982; Cartea et al. 2009; Badenes-Pérez et al. 2017). The cabbage whitefly is a polyphagous insect (Martin et al. 2000), while the small white is a specialist that feeds on crucifers (Verschaffelt 1910; Renwick 2001; Badenes-Pérez 2023). For the cabbage whitefly, glucosinolate content in cruciferous host plants does not seem to be a deterrent factor, rather the opposite, as higher content of the aliphatic glucosinolate sinigrin and total glucosinolate content have been shown to be associated to higher densities of cabbage whitefly in Brassica oleracea L. (Brassicaceae) (Newton et al. 2010; Hondelmann et al. 2020). Larvae of the small white possess a nitrile-specifier protein that directs glucosinolate hydrolysis to the formation of the less toxic nitriles, which allows them to feed on glucosinolate-containing crucifers (Wittstock et al. 2004; Agerbirk et al. 2010; Jeschke et al. 2016; Mesimeri et al. 2024). Glucosinolates and their glucosinolates products can affect plant resistance to small white larvae (Giamoustaris and Mithen 1995; Agrawal and Kurashige 2003; De Vos et al. 2008; Santolamazza-Carbone et al. 2016; Badenes-Pérez 2023) and can also act as host recognition cues for small white butterflies prior to ovipositing on plants (Renwick et al. 1992; Müller et al. 2010; Badenes-Pérez 2023). When making intraspecific comparison among plant accessions of Arabidosis thaliana L. and Brassica oleracea L. (Brassicaceae) that had different glucosinolate content, small white butterflies preferred to oviposit on accessions with higher total glucosinolate content and lower concentrations of certain aliphatic glucosinolates (Poelman et al. 2009; Müller et al. 2010; Coolen et al. 2022). Ovipositing small white females prefer indol-3-ylmethylglucosinolate and 2-phenylethylglucosinolate, an indolic and a benzenic glucosinolate, respectively, over allylglucosinolate, an aliphatic glucosinolate (Traynier and Truscott 1991; Renwick et al. 1992; Städler et al. 1995). Indolic glucosinolates are more important than aliphatic glucosinolates in determining small white oviposition preference (Huang and Renwick 1994; Badenes-Pérez 2023). Aliphatic glucosinolates can be detrimental in the ovipositional preference of the small white (Coolen et al. 2022).
Barbarea spp. have different glucosinolate concentrations (Agerbirk et al. 2003b; Badenes-Pérez et al. 2014). In the G and P types, the dominant glucosinolates are glucobarbarin and epiglucobarbarin, respectively, which are both benzenic glucosinolates, but the P type contains higher total glucosinolate content than the G type (Agerbirk et al. 2003b; Badenes-Pérez et al. 2014), which could affect oviposition preference in the small white. Unlike the diamondback moth, the small white can use the G type as a host plant (van Leur et al. 2008; Badenes-Pérez 2023).
The use of trap crops and insectary plants to manage a particular insect pest species can be affected by how susceptible they are to other pests, especially if the purpose is to eliminate or reduce pesticide use. For this reason, Barbarea spp. that show resistance to other insect pests besides the main target pest should be preferred over the ones that are more susceptible to other pests. In this study, Barbarea rupicola, B. verna, and the G and P types of B. vulgaris were tested for their susceptibility to the cabbage whitefly, while the oviposition preference of the small white was tested with the G and P types.
Materials and methods
Seeds of B. rupicola and B. verna were purchased at B & T World Seeds, Aigues-Vives, France, and Johnny´s Selected Seeds, Albion, ME, USA, respectively. Seeds of the G and P types were originally from Denmark; they were donated by Drs. Niels Agerbirk and Jens K. Nielsen and were later multiplied in Spain (see accessions B44 and B4 for G- and P-type seeds, respectively, in Agerbirk et al. (2003a, b)). Plants used in the experiments were grown in a peat moss substrate that included 1 kg of tref base fertilizer 17–10-14 per 70 l of substrate (Jiffy Tref GO V4, Jiffy Group, the Netherlands). Plants were grown in 15-cm pots. All plants grew as rosettes at a vegetative (pre-flowering stage) and B. rupicola plants appeared to be smaller than the plants of the other Barbarea spp. (personal observation).
Susceptibility of Barbarea plants to cabbage whitefly and powdery mildew
The experiment was conducted to test differences in resistance and susceptibility to cabbage whitefly among B. rupicola, B. verna, and G- and P-type B. vulgaris plants. The experiment was conducted in the greenhouse at 25 ± 3 ºC at the Institute of Agricultural Sciences in Madrid, Spain. The experiment relied on outbreaks and background infection pressure of whitefly and mildew populations that were naturally occurring in the greenhouse when the experiment was conducted. Plants were grown in the same greenhouse all the time and they were randomly located on a bench at the greenhouse and the minimum distance between adjacent plants was 15 cm. A total of 24 plants of each species and type were used. Plants were 12 weeks old when the observations were made. The whole plant was inspected and observations recorded the presence (any amount)/absence of any life stages of the cabbage whitefly in each plant. During the experiment, the percentage of plants affected by powdery mildew was also recorded.
Oviposition preference of the cabbage white butterfly for G- and P-type B. vulgaris
The experiment was conducted to test if female small white butterflies showed an ovipositional preference for either the G or the P type. Small white butterflies were collected in Jena, Germany, and were successively reared on cabbage plants. Insects were reared in environmental growth chambers (16:8 h light:dark, 21 ± 2º C and 55 ± 5 RH). Plants were 7 weeks old when the experiments were conducted. Two-choice oviposition experiments (i.e., one plant of the G type versus one plant of the P type) were conducted in 32.5 × 32.5 × 32.5 cm polyester cages that had a 96 × 26 mesh (MegaView Science Education Services Co., Ltd., Taichung, Taiwan). Five cages were used, each of which was considered a replicate. Two pairs of small white butterflies (two females and two males) were released in each cage and a small plastic cup with a 10% sugar solution on cotton was placed in the middle of the cage as a food source for the butterflies. Two days after releasing the butterflies, the number of eggs on the plants was counted. The location of eggs, either on the abaxial or the adaxial leaf sides of each plant, was also recorded in order to determine if the small white had a particular oviposition preference for either abaxial or adaxial leaf surfaces in the G and P types. Number of eggs on abaxial versus adaxial leaf surfaces was compared within each plant type. Additionally, the rate of abaxial/adaxial oviposition was calculated for each plant as the number of eggs laid on the abaxial leaf side divided by the number of eggs laid on the adaxial leaf side. This abaxial/adaxial oviposition rate was used to compare the two plant types in terms of oviposition preference for either abaxial or adaxial leaf surfaces. On abaxial and adaxial leaf surfaces in P-type plants, the number of trichomes was counted on leaves of different sizes, with maximum leaf widths ranging from 10 to 61 mm, by randomly selecting three leaves in each of four plants. A measure of trichome density was obtained by dividing the number of trichomes on each leaf sampled by the maximum leaf width of the same leaf. The maximum leaf width (mm) was measured in the large end-lobe of the compounded leaf and also coincided with the maximum leaf width of the whole compound leaf.
Statistical analysis
Data comparing percentage of plants with presence of cabbage whitefly and powdery mildew were analyzed using a one-tailed, two-sample test of proportions using STATA® version 15.1 with significance at P ≤ 0.05. To compare the oviposition preference of the small white between G- and P-type plants and between abaxial and adaxial leaf surfaces within the same plant type, data were analyzed using ANOVA with SPSS®. Prior to analysis, the suitability of the data for ANOVA was checked with the Kolmogorov–Smirnov normality test and the Levene test using SPSS®. The rates of abaxial/adaxial oviposition of the small white on G- and P-type plants and the number of trichomes/leaf width in P-type plants, data were analyzed using the Mann–Whitney U-test with SPSS®.
Results
Susceptibility of Barbarea plants to cabbage whitefly and powdery mildew
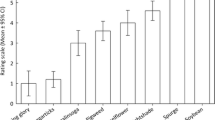
The percentage of plants with presence of cabbage whitefly was 50% in the G type, 8.3% in B. verna, and no infestation at all in B. rupicola and the P type (Fig. 1). Differences in the presence of cabbage whitefly were statistically significant when comparing the G type with B. verna (z = 3.18; P ≤ 0.001), with B. rupicola (z = 4.00; P ≤ 0.001), and with the P type (z = 4.00; P ≤ 0.001). In contrast, 95.8% of the P-type plants showed symptoms of powdery mildew, while the G type and the other Barbarea spp. were unaffected by this fungus and this difference in infection between the P type and the other Barbarea spp. and types was statistically significant (z = 3.18; P ≤ 0.001). Plants affected by cabbage whitefly and powdery mildew did not appear stunted, indicating that infection was not severe.
Oviposition preference of the small white for G- and P-type B. vulgaris
Small white female butterflies showed no oviposition preference between G- and P-type plants (F1, 9 = 0.52, P = 0.491) (P ≤ 0.05) (Fig. 2). In G-type plants, small white showed no significant differences in oviposition on abaxial and adaxial leaf surfaces (F1, 9 = 1.19, P = 0.306), while these differences were significant in the case of P-type abaxial versus adaxial surfaces (F1, 9 = 8.58, P = 0.019) (Fig. 2). When comparing the rate of abaxial/adaxial leaf surface oviposition between the G and P types, small white oviposition on P-type plants tended to occur on the adaxial leaf side and the rate of abaxial/adaxial oviposition was lower on the P type than on the G type (TS = 2.00, P = 0.032). The value of trichome number/leaf width was slightly smaller on the adaxial (3.27 ± 0.82) than on the abaxial (4.38 ± 1.18) leaf surface of P-type plants, but this difference was not statistically significant (TS = 56.00, P = 0.378).
Discussion
This study shows that B. rupicola, B. verna, and the P type are significantly less attractive to cabbage whitefly than the G type. This indicates that in locations were cabbage whitefly is a prevalent economic pests, B. verna, which can also be used as a dead-end trap crop for diamondback moth (Badenes-Pérez et al. 2014), could be chosen over the G type as trap crop. As the presence of trichomes in some Brassica spp. has been associated with resistance to cabbage whitefly, the presence of trichomes in the P type could be responsible for the antixenotic resistance observed in this plant type (Ramsey and Ellis 1996; Pelgrom et al. 2015). Further research is needed to understand the mechanisms of antixenotic resistance to cabbage whitefly observed in B. rupicola, B. verna, and the P type. The apparent glossiness of the G type, B. rupicola, and B. verna seems to indicate that these plant types contain lower amounts of leaf epicuticular wax on the leaf surface than the P type (Badenes-Pérez, personal observation). Leaf cuticular wax has been shown to affect plant susceptibility to whiteflies in cotton (Ali et al. 2021). However, wax removal in two cabbage varieties did not affect cabbage whitefly infestation (Broekgaarden et al. 2012).
Further research is needed to test if P-type plants would be equally affected by powdery mildew in the field compared to greenhouse conditions. Several studies have shown that powdery mildew is an emerging threat to some Brassica crops under climate warming conditions, but it is unknown if the P type would be as severely affected as other Brassicaceae species (Enright and Cipollini 2007; Barbetti et al. 2012; Uloth et al. 2018; Runno-Paurson et al. 2021). The fact that the P type has larger leaves than the other Barbarea spp. and types tested (Badenes-Pérez and López-Pérez 2018) might have also affected powdery mildew infection because larger leaves can catch more water and retain it for longer time (Bradley et al. 2003). Trichomes can also capture fungal spores and be a preferred site for fungal infection (Kim 2019). It is also unknown if the presence of powdery mildew had any effect on the absence of cabbage whitefly in P-type plants. Fungal infection can induce plant secondary metabolites and influence subsequent attraction and resistance to insects (Abdel-Farid et al. 2009; Aghajanzadeh et al. 2023; Jindřichová et al. 2024). Testing if the presence of powdery mildew has any effect on the absence of cabbage whitefly infection in P-type plants would require either using a fungicide, which could also affect insect infestation (Sánchez-Bayo 2021; Margus et al. 2023), or conducting the cabbage whitefly infection tests in environmental conditions less conducive to powdery mildew infection than the ones tested in these experiments. In the P-type plants tested here, not a single cabbage whitefly was seen, even in leaves that showed no powdery mildew infection. P-type plants affected by powdery mildew were not stunted nor had all their leaves with visible infection by this fungus. Thus, mildew infection was not as severe as to completely prevent cabbage whitefly from using P-type plants as a host. Absence of cabbage whitefly in the P type could be due to the marked preference of this insect for other host plants, like the G type and B. verna.
There was no difference in ovipositional preference by the small white between G- and P-type plants with different trichome densities and benzenic glucosinolate content. In a different study with two other chemotypes of B. vulgaris that have different benzenic glucosinolate content as dominant glucosinolates, small white also did not show any oviposition preference for any of the chemotypes (van Leur et al. 2008). In the present study, differences in small white oviposition preference between the G and P types occurred at the within-plant level, where small white preferred ovipositing on the adaxial leaf surfaces on P-type plants and showed no preference for either abaxial or adaxial leaf surfaces on G-type plants. In a different study with 16 different species of plants in the order Brassicales, this oviposition preference of small white for adaxial leaf surfaces was shown for two other species, Arabidopsis thaliana L. and Iberis amara L., while for the other plant species tested, the differences between abaxial and adaxial oviposition were not significant. Glucosinolate content on adaxial leaf surfaces was found to be lower than on abaxial leaf surfaces in B. rupicola and B. verna, showing no significant differences on the G type (Badenes-Pérez et al. 2011). However, the content of glucosinolates on the leaf surfaces of P-type plants is unknown and using gum arabic to mechanically remove surface waxes and determine glucosinolate content was not possible because of the removal of fragments of plant tissue with the gum arabic peelings (Badenes-Pérez, personal observation). Thus, it is unknown if the oviposition preference of the small white for the adaxial leaf surface of the P type could be due to the differences in glucosinolate content. Although trichome density was slightly lower on adaxial than on abaxial leaf surfaces in the leaves sampled, this difference was not statistically significant, so an oviposition preference for lower trichome density was not found in the study. The fact that there is no oviposition preference between the P- and G-type plants also seems to indicate that trichome density is not a major factor in the oviposition preference of the small white. Preference for adaxial versus abaxial oviposition preference may affect the management of the small white because parasitism can be higher for small white larvae located on the adaxial side compared to the abaxial side of leaves (Tagawa et al. 2008). Furthermore, some insecticide sprayers deposit more insecticide on the adaxial than on the abaxial leaf side (Maski and Durairaj 2010). Oviposition on the adaxial leaf side, which is more visible to birds and other predators, could also increase predation (Baker 1970; Schmaedick and Shelton 1999) and susceptibility to dislodgement by rainfall, as it happens in P. xylostella (Rahman et al. 2019).
When trap crops and insectary plants are deployed to manage a particular insect pest species, they can be affected by other pests, which could reduce their effectiveness as trap crops. Barbarea spp. that show resistance to other insect pests besides the main target pest should be preferred. Both the cabbage whitefly and the small white are common pests in cruciferous crops. This study shows that different species and types of Barbarea show different levels of antixenotic resistance to cabbage whitefly and different patterns of oviposition preference in the small white. For this reason, depending on the relative economic importance of pests, different Barbarea spp. and types could be chosen as trap crops for P. xylostella. Further research is needed to understand the mechanisms of antixenotic resistance to cabbage whitefly in Barbarea spp. and types, which could be used as a source of resistance to this pest in plant breeding programs.
Data availability
The data that support the findings of this study are available from the author upon reasonable request.
References
Abdel-Farid IB, Jahangir M, van den Hondel CAMJJ et al (2009) Fungal infection-induced metabolites in Brassica rapa. Plant Sci 176:608–615. https://doi.org/10.1016/j.plantsci.2009.01.017
Agerbirk N, Olsen CE, Nielsen JK (2001) Seasonal variation in leaf glucosinolates and insect resistance in two types of Barbarea vulgaris ssp. arcuata. Phytochemistry 58:91–100. https://doi.org/10.1016/S0031-9422(01)00151-0
Agerbirk N, Olsen CE, Bibby BM et al (2003a) A saponin correlated with variable resistance of Barbarea vulgaris to the diamondback moth Plutella xylostella. J Chem Ecol 29:1417–1433. https://doi.org/10.1023/A:1024217504445
Agerbirk N, Orgaard M, Nielsen JK (2003b) Glucosinolates, flea beetle resistance, and leaf pubescence as taxonomic characters in the genus Barbarea (Brassicaceae). Phytochemistry 63:69–80. https://doi.org/10.1016/S0031-9422(02)00750-1
Agerbirk N, Olsen CE, Poulsen E et al (2010) Complex metabolism of aromatic glucosinolates in Pieris rapae caterpillars involving nitrile formation, hydroxylation, demethylation, sulfation, and host plant dependent carboxylic acid formation. Insect Biochem Mol Biol 40:126–137. https://doi.org/10.1016/j.ibmb.2010.01.003
Agerbirk N, Olsen CE, Heimes C et al (2015) Multiple hydroxyphenethyl glucosinolate isomers and their tandem mass spectrometric distinction in a geographically structured polymorphism in the crucifer Barbarea vulgaris. Phytochemistry 115:130–142. https://doi.org/10.1016/j.phytochem.2014.09.003
Aghajanzadeh TA, Watanabe M, Tohge T et al (2023) Necrotrophic fungal infection affects indolic glucosinolate metabolism in Brassica rapa. Acta Physiol Plant 45:64. https://doi.org/10.1007/s11738-023-03546-3
Agrawal AA, Kurashige NS (2003) A role for isothiocyanates in plant resistance against the specialist herbivore Pieris rapae. J Chem Ecol 29:1403–1415. https://doi.org/10.1023/A:1024265420375
Ali MA, Khan MAU, Rao AQ et al (2021) Biochemical evidence of epicuticular wax compounds involved in cotton-whitefly interaction. PLoS ONE 16:1–14. https://doi.org/10.1371/journal.pone.0250902
Badenes-Pérez FR (2019) Trap crops and insectary plants in the order Brassicales. Ann Entomol Soc Am 112:318–329. https://doi.org/10.1093/aesa/say043
Badenes-Pérez FR (2023) Plant glucosinolate content and host-plant preference and suitability in the small white butterfly (Lepidoptera: Pieridae) and comparison with another specialist lepidopteran. Plants 12:2148. https://doi.org/10.3390/plants12112148
Badenes-Pérez FR, López-Pérez JA (2018) Resistance and susceptibility to powdery mildew, root-knot nematode, and western flower thrips in two types of winter cress (Brassicaceae). Crop Prot 110:41–47. https://doi.org/10.1016/j.cropro.2018.03.015
Badenes-Pérez FR, Shelton AM, Nault BA (2004) Evaluating trap crops for diamondback moth, Plutella xylostella (Lepidoptera: Plutellidae). J Econ Entomol 97:1365–1372. https://doi.org/10.1093/jee/97.4.1365
Badenes-Pérez FR, Shelton AM, Nault BA (2005) Using yellow rocket as a trap crop for diamondback moth (Lepidoptera: Plutellidae). J Econ Entomol 98:884–890. https://doi.org/10.1603/0022-0493-98.3.884
Badenes-Pérez FR, Reichelt M, Gershenzon J, Heckel DG (2011) Phylloplane location of glucosinolates in Barbarea spp. (Brassicaceae) and misleading assessment of host suitability by a specialist herbivore. New Phytol 189:549–556. https://doi.org/10.1111/j.1469-8137.2010.03486.x
Badenes-Pérez FR, Reichelt M, Gershenzon J, Heckel DG (2014) Using plant chemistry and insect preference to study the potential of Barbarea (Brassicaceae) as a dead-end trap crop for diamondback moth (Lepidoptera: Plutellidae). Phytochemistry 98:137–144. https://doi.org/10.1016/j.phytochem.2013.11.009
Badenes-Pérez FR, Márquez BP, Petitpierre E (2017) Can flowering Barbarea spp. (Brassicaceae) be used simultaneously as a trap crop and in conservation biological control? J Pest Sci 90:623–633. https://doi.org/10.1007/s10340-016-0815-y
Baker RR (1970) Bird predation as a selective pressure on the immature stages of the cabbage butterflies, Pieris rapae and P. brassicae. J Zool 162:43–59. https://doi.org/10.1111/j.1469-7998.1970.tb01256.x
Barbetti MJ, Banga SS, Salisbury PA (2012) Challenges for crop production and management from pathogen biodiversity and diseases under current and future climate scenarios–case study with oilseed Brassicas. Field Crop Res 127:225–240. https://doi.org/10.1016/j.fcr.2011.11.021
Bradley DJ, Gilbert GS, Parker IM (2003) Susceptibility of clover species to fungal infection: the interaction of leaf surface traits and environment. Am J Bot 90:857–864. https://doi.org/10.3732/ajb.90.6.857
Broekgaarden C, Riviere P, Steenhuis G et al (2012) Phloem-specific resistance in Brassica oleracea against the whitefly Aleyrodes proletella. Entomol Exp Appl 142:153–164. https://doi.org/10.1111/j.1570-7458.2011.01210.x
Cartea ME, Padilla G, Vilar M, Velasco P (2009) Incidence of the major brassica pests in northwestern spain. J Econ Entomol 102:767–773. https://doi.org/10.1603/029.102.0238
Christensen S, Heimes C, Agerbirk N et al (2014) Different geographical distributions of two chemotypes of Barbarea vulgaris that differ in resistance to insects and a pathogen. J Chem Ecol 40:491–501. https://doi.org/10.1007/s10886-014-0430-4
Christensen S, Sørensen H, Munk KR, Hauser TP (2016) A hybridisation barrier between two evolutionary lineages of Barbarea vulgaris (Brassicaceae) that differ in biotic resistances. Evol Ecol 30:887–904. https://doi.org/10.1007/s10682-016-9858-z
Christensen S, Enge S, Jensen KR et al (2018) Different herbivore responses to two co-occurring chemotypes of the wild crucifer Barbarea vulgaris. Arthropod-Plant Interac. https://doi.org/10.1007/s11829-018-9633-x
Coolen S, van Dijen M, van Pelt JA, van Loon JJA, Pieterse CM, van Wees SC (2022) Genome-wide association study reveals WRKY42 as novel player in oviposition preference of Pieris butterflies. J Exp Bot 23:501. https://doi.org/10.1093/jxb/erac501
De Vos M, Kriksunov KL, Jander G (2008) Indole-3-acetonitrile production from indole glucosinolates deters oviposition by Pieris rapae. Plant Physiol 146:916–926. https://doi.org/10.1104/pp.107.112185
Enright SM, Cipollini D (2007) Infection by powdery mildew Erysiphe cruciferarum (Erysiphaceae) strongly affects growth and fitness of Alliaria petiolata (Brassicaceae). Am J Bot 94:1813–1820. https://doi.org/10.3732/ajb.94.11.1813
Giamoustaris A, Mithen R (1995) The effect of modifying the glucosinolate content of leaves of oilseed rape (Brassica napus ssp. oleifera) on its interaction with specialist and generalist pests. Ann of Appl Biol 126:347–363. https://doi.org/10.1111/j.1744-7348.1995.tb05371.x
Hauser TP, Toneatto F, Nielsen JK (2012) Genetic and geographic structure of an insect resistant and a susceptible type of Barbarea vulgaris in western Europe. Evol Ecol 26:611–624. https://doi.org/10.1007/s10682-011-9515-5
Hauser TP, Christensen S, Kuzina V et al (2021) Effects of a saponin-based insect resistance and a systemic pathogen resistance on field performance of the wild crucifer Barbarea vulgaris. Arthropod-Plant Interact 15:683–698. https://doi.org/10.1007/s11829-021-09858-w
Heimes C, Thiele J, van Mölken T, Hauser TP (2015) Interactive impacts of a herbivore and a pathogen on two resistance types of Barbarea vulgaris (Brassicaceae). Oecologia 177:441–452. https://doi.org/10.1007/s00442-014-3113-5
Heimes C, Agerbirk N, Sorensen H et al (2016) Ecotypic differentiation of two sympatric chemotypes of Barbarea vulgaris (Brassicaceae) with different biotic resistances. Plant Ecol 217:1055–1068. https://doi.org/10.1007/s11258-016-0631-8
Hondelmann P, Paul C, Schreiner M, Meyhöfer R (2020) Importance of antixenosis and antibiosis resistance to the cabbage whitefly (Aleyrodes proletella) in Brussels sprout cultivars. Insects 11:56. https://doi.org/10.3390/insects11010056
Huang X, Renwick JAA (1994) Relative activities of glucosinolates as oviposition stimulants for Pieris rapae and P. napi oleracea. J Chem Ecol 20:1025–1037. https://doi.org/10.1007/bf02059739
Idris AB, Grafius E (1994) The potential of using Barbarea vulgaris in insecticide-resistant diamondback moth management. Res Pest Manag Newsl 6:7–8
Idris AB, Grafius E (1995) Wildflowers as nectar sources for Diadegma insulare (Hymenoptera: Ichneumonidae), a parasitoid of diamondback moth (Lepidoptera: Yponomeutidae). Environ Entomol 24:1726–1735. https://doi.org/10.1093/ee/24.6.1726
Jeschke V, Gershenzon J, Vassão DG (2016) Chapter Eight - Insect detoxification of glucosinolates and their hydrolysis products. In: Kopriva S (ed) Advances in Botanical Research. Academic Press, pp 199–245
Jindřichová B, Rubil N, Rezek J, Ourry M, Hauser TP, Burketová L (2024) Does fungal infection increase the palatability of oilseed rape to insects? Pest Manag Sci 80(5):2480–2494. https://doi.org/10.1002/ps.7998
Kim KW (2019) Plant trichomes as microbial habitats and infection sites. Eur J Plant Pathol 154:157–169. https://doi.org/10.1007/s10658-018-01656-0
Kuzina V, Ekstrøm CT, Andersen SB et al (2009) Identification of defense compounds in Barbarea vulgaris against the herbivore Phyllotreta nemorum by an ecometabolomic approach. Plant Physiol 151:1977–1990. https://doi.org/10.1104/pp.109.136952
Kuzina V, Nielsen JK, Augustin JM et al (2011) Barbarea vulgaris linkage map and quantitative trait loci for saponins, glucosinolates, hairiness and resistance to the herbivore Phyllotreta nemorum. Phytochemistry 72:188–198. https://doi.org/10.1016/j.phytochem.2010.11.007
Lu JH, Liu SS, Shelton AM (2004) Laboratory evaluations of a wild crucifer Barbarea vulgaris as a management tool for the diamondback moth Plutella xylostella (Lepidoptera: Plutellidae). Bull Entomol Res 94:509–516. https://doi.org/10.1079/BER2004328
Margus A, Saifullah S, Kankare M, Lindström L (2023) Fungicides modify pest insect fitness depending on their genotype and population. Sci Rep 13:17879. https://doi.org/10.1038/s41598-023-44838-5
Martin JH, Mifsud D, Rapisarda C (2000) The whiteflies (Hemiptera: Aleyrodidae) of Europe and the Mediterranean Basin. Bull Entomol Res 90:407–448. https://doi.org/10.1017/S0007485300000547
Maski D, Durairaj D (2010) Effects of charging voltage, application speed, target height, and orientation upon charged spray deposition on leaf abaxial and adaxial surfaces. Crop Prot 29:134–141. https://doi.org/10.1016/j.cropro.2009.10.006
Mesimeri I-D, Revelou P-K, Constantinou-Kokotou V, Kokotou MG (2024) Determination of phenethyl isothiocyanate, erucin, iberverin, and erucin nitrile concentrations in healthy and Pieris rapae-infected broccoli tissues using gas chromatography-mass spectrometry. Chemosensors 12:16. https://doi.org/10.3390/chemosensors12010016
Müller R, de Vos M, Sun J et al (2010) Differential effects of indole and aliphatic glucosinolates on lepidopteran herbivores. J Chem Ecol 36:905–913. https://doi.org/10.1007/s10886-010-9825-z
Newton E, Bullock J, Hodgson D (2010) Temporal consistency in herbivore responses to glucosinolate polymorphism in populations of wild cabbage (Brassica oleracea). Oecologia 164:689–699. https://doi.org/10.1007/s00442-010-1702-5
Nielsen JK, Nagao T, Okabe H, Shinoda T (2010a) Resistance in the plant, Barbarea vulgaris, and counter-adaptations in flea beetles mediated by saponins. J Chem Ecol 36:277–285. https://doi.org/10.1007/s10886-010-9758-6
Nielsen NJ, Nielsen J, Staerk D (2010b) New resistance-correlated saponins from the insect-resistant crucifer Barbarea vulgaris. J Agric Food Chem 58:5509–5514. https://doi.org/10.1021/jf903988f
Pelgrom KTB, Broekgaarden C, Voorrips RE et al (2015) Host plant resistance towards the cabbage whitefly in Brassica oleracea and its wild relatives. Euphytica 202:297–306. https://doi.org/10.1007/s10681-014-1306-y
Poelman EH, van Dam N, van Loon JJA et al (2009) Chemical diversity in Brassica oleracea affects biodiversity of insect herbivores. Ecology 90:1863–1877. https://doi.org/10.1890/08-0977.1
Rahman MM, Zalucki MP, Furlong MJ (2019) Diamondback moth egg susceptibility to rainfall: effects of host plant and oviposition behavior. Entomol Exp Appl 167:701–712. https://doi.org/10.1111/eea.12816
Ramsey AD, Ellis PR (1996) Resistance in wild brassicas to the cabbage whitefly, Aleyrodes proletella. Acta Hort 407:507–514
Renwick JAA (2001) Variable diets and changing taste in plant–insect relationships. J Chem Ecol 27:1063–1076. https://doi.org/10.1023/a:1010381509601
Renwick JAA, Radke CD, Sachdev-Gupta K, Städler E (1992) Leaf surface chemicals stimulating oviposition by Pieris rapae (Lepidoptera: Pieridae) on cabbage. Chemoecology 3:33–38. https://doi.org/10.1007/bf01261454
Runno-Paurson E, Lääniste P, Eremeev V et al (2021) Powdery mildew (Erysiphe cruciferarum) evaluation on oilseed rape and alternative cruciferous oilseed crops in the northern Baltic region in unusually warm growing seasons. Acta Agric Scand, Sec B—Soil Plant Sci 71:443–452. https://doi.org/10.1080/09064710.2021.1914714
Sánchez-Bayo F (2021) Indirect effect of pesticides on insects and other arthropods. Toxics 9:177. https://doi.org/10.3390/toxics9080177
Santolamazza-Carbone S, Sotelo T, Velasco P, Cartea ME (2016) Antibiotic properties of the glucosinolates of Brassica oleracea var. acephala similarly affect generalist and specialist larvae of two lepidopteran pests. J Pest Sci 89:195–206. https://doi.org/10.1007/s10340-015-0658-y
Schmaedick MA, Shelton AM (1999) Experimental evaluation of arthropod predation on Pieris rapae (Lepidoptera: Pieridae) eggs and larvae in cabbage. Environ Entomol 28:439–444. https://doi.org/10.1093/ee/28.3.439
Shelton AM, Badenes-Pérez FR (2006) Concepts and applications of trap cropping in pest management. Annu Rev Entomol 51:285–308. https://doi.org/10.1146/annurev.ento.51.110104.150959
Shelton AM, Nault BA (2004) Dead-end trap cropping: a technique to improve management of the diamondback moth, Plutella xylostella (Lepidoptera: Plutellidae). Crop Prot 23:497–503. https://doi.org/10.1016/j.cropro.2003.10.005
Shelton AM, Andaloro JT, Barnards J (1982) Effects of cabbage looper, imported cabbageworm, and diamondback moth on fresh market and processing cabbage. J Econ Entomol 75:742–745. https://doi.org/10.1093/jee/75.4.742
Städler E, Renwick JAA, Radke CD, Sachdev-Gupta K (1995) Tarsal contact chemoreceptor response to glucosinolates and cardenolides mediating oviposition in Pieris rapae. Physiol Entomol 20:175–187. https://doi.org/10.1111/j.1365-3032.1995.tb00814.x
Tagawa J, Matsushita A, Watanabe T (2008) Leaf surface preference in the cabbage worm, Pieris rapae crucivora, and parasitism by the gregarious parasitoid Cotesia glomerata. Entomol Exp Appl 129:37–43. https://doi.org/10.1111/j.1570-7458.2008.00750.x
Traynier RMM, Truscott RJW (1991) Potent natural egg-laying stimulant for cabbage butterfly Pieris rapae. J Chem Ecol 17:1371–1380. https://doi.org/10.1007/bf00983770
Uloth MB, You MP, Barbetti MJ (2018) Plant age and ambient temperature: significant drivers for powdery mildew (Erysiphe cruciferarum) epidemics on oilseed rape (Brassica napus). Plant Pathol 67:445–456. https://doi.org/10.1111/ppa.12740
van Leur H, Vet LEM, van der Puten WH, van Dam NM (2008) Barbarea vulgaris glucosinolate phenotypes differentially affect performance and preference of two different species of lepidopteran herbivores. J Chem Ecol 34:121–131. https://doi.org/10.1007/s10886-007-9424-9
van Mölken T, Heimes C, Hauser TP, Sundelin T (2014) Phylogeny of an Albugo sp. infecting Barbarea vulgaris in Denmark and its frequency of symptom development in natural populations of two evolutionary divergent plant types. Fungal Biol 118:340–347. https://doi.org/10.1016/j.funbio.2014.01.008
Verschaffelt E (1910) The cause determining the selection of food in some herbivorous insects. Proc Acad Sci Amsterdam 13:536–542
Wittstock U, Agerbirk N, Stauber EJ et al (2004) Successful herbivore attack due to metabolic diversion of a plant chemical defense. Proc Natl Acad Sci USA 101:4859–4864. https://www.pnas.org/doi/full/10.1073/pnas.0308007101
Acknowledgements
I am grateful to Beatriz Parrado Márquez, for technical assistance, and to Natascha Rauch and Bettina Raguschke for the help in rearing small white insects. I also thank Drs. David G. Heckel and Johnathan Gershenzon for various types of help and support in this research and Drs. Niels Agerbirk and Jens K. Nielsen for providing B. vulgaris seeds. This research was supported by the Spanish Ministry of Science and Innovation (AGL2010-18151) and the Max Planck Society.
Funding
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The author declares no conflict of interest.
Additional information
Handling Editor: Jaime Pinero.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Badenes-Pérez, F.R. Resistance and susceptibility of different Barbarea (Brassicaceae) species and types to cabbage whitefly (Hemiptera: Aleyrodidae) and cabbage white butterfly (Lepidoptera: Pieridae). Arthropod-Plant Interactions (2024). https://doi.org/10.1007/s11829-024-10081-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11829-024-10081-6