Abstract
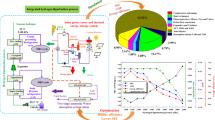
As the movement for carbon neutrality spreads around the world, research on hydrogen energy is also being actively conducted. In the hydrogen value chain, liquefaction is a particularly energy-intensive process. Although the operating energy of the hydrogen liquefaction process can vary greatly depending on the season or regional cooling water temperature, previous studies have not taken this into account. In this study, we quantitatively identify the effect of the change in cooling water temperature on exergy destruction, specific energy consumption (SEC), and coefficient of performance (COP) of the liquefaction process. In addition, we propose a design improvement to reduce exergy destruction with the exergy analysis of multi-stream heat exchangers. A new design (auxiliary process) that reduces exergy destruction is proposed by analyzing the device where energy destruction occurs the most. When the cooling water temperature increases from 20 °C to 35 °C, there is a tendency for SEC and exergy destruction to increase and the COP to decrease. The new design with an auxiliary process shows a decrease in SEC and a reduced rate of increase in SEC in response to the increase in cooling water temperature. The base case without the auxiliary cycle shows that the SEC with cooling water of 35 °C is 14.66% greater than that with cooling water of 20 °C, while the proposed process shows the rate of increase of 9.70%. This means that adding the auxiliary cycle can improve the energy efficiency and increase robustness to variations in ambient conditions.
Similar content being viewed by others
Abbreviations
- A:
-
heat transfer area
- C p :
-
specific heat capacity [kJ/kmole °C]
- CR:
-
compression ratio
- Ex:
-
rate of exergy [kW]
- I:
-
irreversibility [kW]
- k:
-
specific heat ratio
- LMTD:
-
logarithmic mean temperature difference
- ρ :
-
liquid density [kg/m3]
- \(\mathop {\rm{m}}\limits^ \cdot\) :
-
molar flow rate [kmole/s]
- P:
-
pressure [bar]
- q:
-
volumetric flow rate [m3/h]
- T:
-
temperature
- U:
-
overall heat transfer coefficient [kW/m3·°C]
- \(\mathop {\rm{W}}\limits^ \cdot\) :
-
work transfer rate [kW]
- x:
-
component mole fraction
- Z:
-
gas compressibility factor
- η :
-
efficiency
- SEC:
-
specific energy consumption
- COM:
-
compressor
- COP:
-
coefficient of performance
- EXP:
-
expander
- CRV:
-
conversion reactor
- HX:
-
heat exchanger
- BHP:
-
brake horsepower
- c:
-
cold
- Ex:
-
exergy
- H:
-
hot
- i:
-
component i
- o:
-
outlet
- P:
-
pump
- s:
-
suction
- °:
-
standard condition
References
R. P. Date, N. O. Asia and M. East, Global and regional coal phase out requirements of the paris agreement: Insights from the IPCC special report on 1.5 C (2019).
F. Wu, N. Huang, F. Zhang, L. Niu and Y. Zhang, Technol. Forecast Soc. Change, 159, 120198 (2020).
A. Kuriyama, K. Tamura and T. Kuramochi, Energy Policy, 130, 328 (2019).
R. Karacan, S. Mukhtarov, İ. Bariş, A. İşleyen and M. E. Yardimci, Energies, 14(10), 2947 (2021).
S. D. Oladipupo, H. Rjoub, D. Kirikkaleli and T. S. Adebayo, Int. J Renew. Energy Dev., 11(1) (2022).
P. P. Edwards, V. L. Kuznetsov and W. I. David, Philos. Trans. Royal Soc. A, 365(1853), 1043 (2007).
A. Midilli, M. Ay, I. Dincer and M. A. Rosen, Renew. Sust. Energ. Rev., 9(3), 255 (2005).
T. N. Veziroğlu and S. Şahi, Energy Convers. Manag., 49(7), 1820 (2008).
K. Cheon and J. Kim, J. Korean Soc. Miner. Energy Resour. Eng., 57(6), 629 (2020).
S. K. Kar, A. S. K. Sinha, R. Bansal, B. Shabani and S. Harichandan, WIREs Energy Environ., 12(1), e457 (2022).
C. Philibert, Perspectives on a hydrogen strategy for the european union, Etudes del’Ifri, Ifri, 51(28), 49 (2020).
B. H. Park and D. H. Lee, Korean J. Chem. Eng., 39(4), 902 (2022).
A. Züttel, Naturwissenschaften, 91(4), 157 (2004).
A. Züttel, Mater. Today, 6(9), 24 (2003).
U. Eberle, M. Felderhoff and F. Schueth, Angew. Chem., Int. Ed., 48(36), 6608 (2009).
S. Krasae-in, J. H. Stang and P. Neksa, Int. J. Hydrogen Energy, 35(10), 4524 (2010).
J. Andersson and S. Grönkvist, Int. J. Hydrogen Energy, 44(23), 11901 (2019).
S. Y Kim and D. K. Choi, Korean Ind. Chem. News, 21(3), 20 (2018).
U. Cardella, L. Decker, J. Sundberg and H. Klein, Int. J. Hydrogen Energy, 42(17), 12339 (2017).
S. Bischoff and L. Decker, AIP Conference Proc., 1218(1), 887 (2010).
A. Tarique, I. Dincer and C. Zamfirescu, Application of scroll expander in cryogenic process of hydrogen liquefaction, In: Progress in exergy, energy, and the environment, Springer, 91 (2014).
R. F. Abdo, H. T. Pedro, R. N. Koury, L. Machado, C. F. Coimbra and M. P. Porto, Energy, 90, 1024 (2015).
K. D. Timmerhaus and T. M. Flynn, Cryogenic process engineering, Springer Science & Business Media (2013).
T. K. Nandi and S. Sarangi, Int. J. Hydrogen Energy, 18(2), 131 (1993).
D. Berstad, G. Skaugen and 0. Wilhelmsen, Int. J. Hydrogen Energy, 46(11), 8014 (2021).
C. W. Park, K. S. Cha, S. G. Lee, C. G. Lee and K. H. Choi, J. Kor. Inst. Gas., 17(1), 67 (2013).
S. Krasae-In, J. H. Stang and P. Neksa, Int. J. Hydrogen Energy, 35(22), 12531 (2010).
S. Krasae-In, A. M. Bredesen, J. H. Stang and P. Neksa, Int. J. Hydrogen Energy, 36(1), 907 (2011).
S. Krasae-In, Int. J. Hydrogen Energy, 39(13), 7015 (2014).
G. Valenti and E. Macchi, Int. J. Hydrogen Energy, 33(12), 3116 (2008).
M. S. Sadaghiani and M. Mehrpooya, Int. J. Hydrogen Energy, 42(9), 6033 (2017).
M. Mehdizadeh-Fard, F. Pourfayaz and A. Maleki, Energy Rep., 7, 174 (2021).
H. Zhang, J. Baeyens, G. Caceres, J. Degreve and Y. Lv, Prog. Energy Combust. Sci., 53, 1 (2016).
H. Ansarinasab, M. Mehrpooya and A. Mohammadi, J. Clean Prod., 144, 248 (2017).
M. Mehrpooya, M. S. Sadaghiani and N. Hedayat, Int. J. Energy Res., 44(3), 1636 (2020).
M. Asadnia and M. Mehrpooya, Int. J. Hydrogen Energy, 42(23), 15564 (2017).
W. Noh, S. Park, J. Kim and I. Lee, Int. J. Energy Res., 46(9), 12926 (2022).
D. Lee, D. Q. Gbadago, Y. Jo, G. Hwang, Y Jo, R. Smith and S. Hwang, Energy Convers. Manag., 245, 114620 (2021).
D. Peng and D. B. Robinson, Ind. Eng. Chem., 15(1), 59 (1976).
R. D. McCarty, J. Hord and H. M. Roder, Selected properties of hydrogen (engineering design data), US Department of Commerce, National Bureau of Standards (1981).
M. Mehrpooya, M.M.M. Sharifzadeh and M.A. Rosen, Energy, 90, 2047 (2015).
M. Picon-Nunez, G. T. Polley and M. Medina-Flores, Appl. Therm. Eng., 22(14), 1643 (2002).
A. Naquash, M. A. Qyyum, S. Min, S. Lee and M. Lee, Energy Convers. Manag., 251, 114947 (2022).
A. Giampaolo, Compressor handbook: Principles and practice, CRC Press (2020).
S. M. Yahya, Turbines compressors and fans, Tata McGraw-Hill Education (2010).
E. Ilisca, Prog. Surf. Sci., 41(3), 217 (1992).
S. Gursu, M. Lordgooei, S. A. Sherif and T. N. Veziroglu, Int. J. Hydrogen Energy, 17(3), 227 (1992).
A. Riaz, M. A. Qyyum, A. Hussain and M. Lee, Int. J. Hydrogen Energy, In Press (2022).
I. Dincer and M. A. Rosen, Thermal energy storage: Systems and applications, John Wiley & Sons (2021).
I. J. Karassik, J. P. Messina, P. Cooper and C. C. Heald, Pump handbook, McGraw-Hill Education (2008).
H. Mahabadipour and H. Ghaebi, Appl. Therm. Eng., 50(1), 771 (2013).
M. Kanoglu, Int. J. Energy Res., 26(8), 763 (2002).
E. Connelly, M. Penev, A. Elgowainy and C. Hunter, Current status of hydrogen liquefaction costs, DOE Hydrogen and Fuel Cells Program Record., 1–10 (2019).
M. Jeong, E. Cho, H. Byun and C. Kang, Korean J. Chem. Eng., 38, 380 (2021).
Acknowledgements
This work is supported by the Korea Institute of Energy Technology Evaluation and Planning (KETEP), granted financial resources from the Ministry of Trade, Industry, and Energy (No. 20227310100010) and by the Korea Agency for Infrastructure Technology Advancement (KAIA) grant funded by the Ministry of Land, Infrastructure and Transport (No. 21ATOG-C162087-01).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Lee, D.H., Yu, S.Y., Yeom, S.Y. et al. Exergy destruction improvement of hydrogen liquefaction process considering variations in cooling water temperature. Korean J. Chem. Eng. 40, 1839–1849 (2023). https://doi.org/10.1007/s11814-023-1480-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11814-023-1480-5