Abstract
In the last few decades, attention on new natural antimicrobial compounds has arisen due to a change in consumer preferences and the increase in the number of resistant microorganisms. Algae are defined as photosynthetic organisms that demonstrate a wide range of adaptability to adverse environmental conditions like temperature extremes, photo-oxidation, high or low salinity, and osmotic stress. Algae are primarily known to produce large amounts of secondary metabolite against various kinds of pathogenic microbes. Among these algae, micro and microalgae of river, lake, and algae of oceanic origin have been reported to have antimicrobial activity against the bacteria and fungi of pathogenic nature. Various polar and non- polar extracts of micro- and macro algae have been used for the suppression of these pathogenic fungi. Apart from these, certain algal derivatives have also been isolated from these having antibacterial and antifungal potential. Among the bioactive molecules of algae, polysaccharides, sulphated polysaccharides, phyco-cyanobilins polyphenols, lectins, proteins lutein, vitamin E, B12 and K1, peptides, polyunsaturated fatty acids and pigments can be highlighted. In the present review, we will discuss the biological activity of these derived compounds as antifungal/ antibacterial agents and their most promising applications. A brief outline is also given for the prospects of these isolated phytochemicals and using algae as therapeutic in the dietary form. We have also tried to answer whether alga-derived metabolites can serve as potential therapeutics for the treatment of SARS-CoV-2 like viral infections too.
Similar content being viewed by others
Introduction
The scientific community is in the search of compounds that can be most effective to fight against novel diseases, one such example of disease is COVID-19 (Tomas et al. 2022). Natural products have been used as therapeutic agents for the treatment of a wide range of illnesses for thousands of years, having an important role in meeting the basic needs of human populations. Since last seven to eight decades’ infectious agents have imperilled the success and achievement of modern era medicine (Levy and Marshall 2004). Antibiotics have revolutionized the system of medicine and was found to be quite effective in prevention and treatment of various kinds of infectious agents (Abdelmohsen et al. 2017). However, the pessimistic impact of antibiotics application against the pathogenic microbes was their ability to develop antimicrobial resistance against it (Abdelmohsen et al. 2017). This in itself would be unproblematic, had it not been for the rapid ability of bacteria to become resistant towards previously debilitating agents. The need for new antibiotics is therefore eminent. Also, such kind of development of antimicrobial resistance has imposed a burden on the health and economics of many developing and under developing countries of the world (Sommer 2014; Fitchett 2015; Tillotson 2015; Tolpeznikaite et al. 2021). To combat the development of drug resistance amongst the microbes some combination-based therapy (viz. antimicrobial-antimicrobial; antimicrobial-adjuvant and drug cocktail) was used over the monotherapy (WHO, 2016). It aided in the prevention and treatment of drug resistant microbes of any infectious disease (Worthington and Melander 2013; Cui et al. 2015; Wells et al. 2015). However, such kind of treatment therapy did not anticipate overcoming the impact of drug resistance. Since prehistoric times natural product has shown their efficiency against the various kinds of diseases. Natural products have been important contributors for antimicrobial drug discovery and development (Pradhan et al. 2022). Plants and plant-products have been used as the source of therapeutics since ancient times due to their ethno-pharmacological characteristics that provided a basic platform for the drug discovery (McRae et al. 2007). The usage of herbal drugs and their market has increased exponentially in the last few decades. According to WHO about 80% of 122 derived plant medicine are used for the ethnopharmacological purpose (Fabricant and Farnsworth 2001). These natural products are quite prevalent in all the sections of society encompassing richer and underprivileged people. Its dissemination in the society is mainly due to its low cost, easy availability in the region, no requirement of medical practitioner, least side effect and many other factors which lure people towards it. As per the present estimates about less than 10% of the total world’s biodiversity is used therapeutically and still many more diverse natural products are awaiting their discovery (McRae et al. 2007; Teasdale et al. 2012). Significant amount of work is done over natural antimicrobials isolated from plant source. However, due to restriction imposed over some plants species as listed in red data book and illegal trading of these natural resources has led to fear of depletion. Furthermore, keeping in view, the risk of environmental impact it is becoming more important to avoid felling of these natural resources for drug development. Replantation of these natural resources may take many years to grow entirely and considering the present scenario of an exponential increase in infections and development of drug resistance have angled scientist to explore other options. Researchers are investigating economically viable natural resources that are not included in the endangered list and can easily regrow in a short duration for continual drug development without affecting the environment.
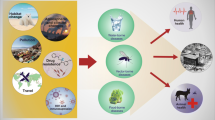
In pursuit of an alternative to these endangered natural resources, many scientists have diverted their attention towards the utilization of the marine environment having the probability of great commercial exploitation globally (Song et al. 2021). The marine environment has an abundant resource of natural products with diverse action in regards to antibacterial, antifungal, antiviral, anti-parasitic, anti-tumour, anti-inflammatory, antioxidant, and immunomodulatory activities. The algae found in the marine environment have an abundant source of natural products which could be exploited for therapeutics and other applications. These algae are found in both forms i.e., prokaryotic, and eukaryotic with a wide variety of habitation ranging from shallow to coastal, and backwater (Bhalodia and Shukla 2011; Song et al. 2021). Many scientific reports have shown that compounds isolated from these marine organisms have both in vitro and in vivo activities against the gram-positive and gram-negative microbes (Pina-Pérez et al. 2017; Ancheeva et al. 2018). Till 2009, a total of 2840 marine species were studied resulting in the isolation of about 20057 metabolites which were published in 7795 scientific articles. Out of 250000 marine species reported to date only 1% of them have been studied and investigated (Blunt et al. 2012) which suggests that the remaining unexplored living organisms comprise biological and chemical treasure. A scheme is proposed for the utilization of bioactive compounds for therapeutic applications (Fig.1). The outline consists of stages proposed for the search, study, systematic examination, and application of potential bioactive molecules isolated from different algal sources. Early studies were concerned mainly with in vitro studies of the action of the compounds and later the focus shifted to emphasise in vivo examination of activities using a very extensive range of screens. These in vitro screens are quicker and cost-effective as they are based on enzymatic activity or on the action of the compounds on cultured cell lines. This also opens new horizons for researchers to develop new molecules of therapeutic potential from algae of the marine sources. Owed to such interesting and pioneering results, algae have made its discrete place in the traditional medicine therapies till date (Geetha Bai and Tuvikene 2021). Marine algae are distributed in a wide range of habitats and entice attention not only due to its taxonomic and ecological perspective but also to produce valuable bioactive components used in the pharmaceutics, foods, and cosmetic industry. In Table1, several types of antimicrobial compounds viz. polysaccharides, polyphenols, fatty acids, pigments, alkaloids, terpenoids, halogenated compounds, proteins and peptides extracted from algae have been compiled. Every class of marine algae ranging from Euglenophyta (Euglenoids), Cryophyte (Golden-brown algae and Diatoms), Pyrrophyta (Fire algae), Chlorophyta (Green algae), Rhodophyta (Red algae), Phaeophyta (Brown algae), Xanthophyta (Yellow-green algae) could be exploited to meet the demands for developing new antimicrobials for prevention and control of drug resistant microbes and correspondingly the demands of foods and cosmetic industries. Different applications have been proposed for these compounds, such as preservatives in the food or cosmetic industries, as antibiotics in the pharmaceutical industry, as anti-biofilm, antifouling, coating in active packaging, prebiotics or in nanoparticles (Greff et al. 2014; Pérez et al. 2016; Carvalho et al. 2019). This mini-review presents the therapeutic potential of alga (micro/macro) and the phytochemicals/bioactive compounds isolated till date with emphasis on the antibacterial, antifungal and antiviral data as well as their applications against drug resistant infectious microbes.
Antibacterial compound isolated from algae
The antibacterial activity of certain natural products is tested in vitro and in vivo against many pathogenic bacteria alone and in combination of certain other nutrients (Pérez et al. 2016; Pradhan et al. 2022). Nonetheless, during last decades an exponential increase in the research of novel antimicrobial compounds from these thallophytes titled scientists to screen more and more compounds which could be exploited likewise. Till now, certain antimicrobial potential algae have been studied that show similar activity to plant-derived natural products both in in vitro and in vivo conditions by agar diffusion methods (Cakmak et al. 2014). Some polar and non-polar solvent extract of algae have been reported to show their potential against the pathogenic bacterial agents. Trentepohlia umbrina an alga in methanol extract has potential against the Klebsiella pneumonia, Aspergillus niger, and Trichoderma barsianum along with the fungal pathogens (Simic et al. 2012). Sargassum wightii and Padina tetrastromatica aqueous extract exhibited its potential against Staphylococcus aureus and Vibrio harveyi (Christabell et al. 2011). Methanolic extract of Chlamydomonas reinhardtii is found to be effective against the bacterial and fungal pathogens including Aspergillus niger, Aspergillus fumigatus and Proteus mirabilis (Alsenani et al. 2020). Methanolic extract of Oscillatoria sancta also reported its potential against the Proteus mirabilis, Proteus vulgaris, and Streptococcus pyogenes (Prakash et al. 2011). Apart from these certain phytoconstituents were also isolated from algae and their antibacterial and antifungal activity were reported. (Kamei et al. 2009) reported a novel antibacterial terpenoid diterpene sargafuran from methanolic extract of marine brown algae Sargassum macrocarpum. The bactericidal nature of this extract was demonstrated through degrading the Propionibacterium acnes using the mechanism of bacterial cells lysis. Certain halogenated sesquiterpenes namely Majapolene B Majapolene A. and Acetylmajapolene A is isolated from Malaysian Laurencia sp against the marine bacteria Chromobacterium violaceum, Proteus mirabilis, Proteus vulgaris, Erwinia sp., Vibrio parahaemolyticus, and V. alginolyticus which evidenced their antibacterial nature in similar mechanism of action to antibiotics (Vairappan et al. 2008). Three new halogenated sesquiterpenes, 10-bromo-7α,8α-expoxychamigr-1-en-3-ol (1), 10-bromo-β-chamigren-8-ol (2), and 10-bromo-3-chlorocupar-5-en-2-ol (3) isolated from the marine red alga Laurencia okamurai at the geological sites coast of Rongcheng, China reported antibacterial activity against Escherichia coli and Staphylococcus aureus using standard agar diffusion assay (Li et al. 2012). The favourite edible seaweed of Hawaiian red alga Asparagopsis taxiformis “limu kohu,” which is the prominent source of organohalogens contains unusual mahorone and 5-bromomahorone which is reported to be active against the marine bacterium Vibrio fisheri (Greff et al. 2014). 4,5,6-tribromo-2-methylsulfinylindole, a new polybrominated indole from Laurencia brongniarii showed anti-bactericidal effect against Enterobacter aerogenes (ATCC 13,048), Salmonella enteritidis (ATCC 13,076), and Serratia marcescens ATCC 25,419 (Fang et al. 2014a, b).
The new bromophycoic acids A–E isolated from the Callophycus sp. from Fijian red alga possesses a range of bactericidal activity against the methicillin-resistant S. aureus and vancomycin resistant Enterococcus facium at Minimum inhibitory concentration of 1.6–6.3µg/mL (Teasdale et al. 2012). Paramuricea clavata, a Mediterranean gorgonian is reported to possess three new brominated metabolites, 2-bromo-N-methyltryptamine, 3-bromo-N-methyltyramine and 6-bromo-N-methyltryptamine which shows their antibacterial activity against the three bacterial strain Pseudoalteromonas sp. D41 and TC8, and Paracoccus sp. 4M6 (Gribble 2015). The brominated alkaloids isolated from the marine sponge Pseudoceratina sp. showed biological activity and presence of four new pseudoceramines A–D. Amongst the previously reported pseudoceramines, Pseudoceramine B inhibits bacterial growth with IC50 40 µM (Yin et al. 2011; Li et al. 2012). Three new brominated imidazoles, 14-O-sulfate massadine, 14-O-methyl massadine, and 3-O-methyl massadine chloride were isolated from the deep-sea Great Australian Bight sponge, Axinella sp. Researchers reported the antibacterial activity of Axinella sp. against the gram-positive bacteria Staphylococcus aureus (ATCC 9144 and 25,923) and B. subtilis (ATCC 6051 and 6633), and the gram-negative bacteria E. coli (ATCC 11,775) and P. aeruginosa (ATCC 10,145) (Zhang et al. 2012; Cui et al. 2015). A novel indole alkaloid isolated from the Okinawan sponge Suberites sp., including nakijinamines A is reported to show antibacterial activity against the S. aureus, B. subtilis, and Micrococcus luteus (França et al. 2014).
Terpenoids are primarily a class of compounds having wide distribution in nature and higher plants, viz. gymnosperms and angiosperms are an abundant source of this. Besides higher plants many thallophytes including marine algae are also an abundant source of it. Terpenoids of marine algae have an immense role in chemical ecology, signal molecules, allochemicals and many more (Yasuhara-Bell and Lu 2010). The major class of terpenoids isolated from marine algae are mainly sesterterpenoids, sesquiterpenoids and meroterpenoids which are antimicrobial in nature. Other potent terpenoids, peyssonoic acid A and B are isolated from a red alga Peyssonnelia sp. and are reported to have microbial activity against Pseudoalteromonas bacteriolytica and Lindra thalassiae (Kurhekar 2020). Similarly, Tiomanene and Acetylmajapolene A and B isolated from the Laurencia sp. are reported to have antimicrobial activity against many pathogenic microbes (Vairappan et al. 2008). Other than terpenoids some phenolic compounds isolated from the marine source are also reported to have antibacterial activity. These phenolics are also widely distributed in the environment and present abundantly in aromatic plants which contain the large group of secondary metabolites. Monodictyoquinone (1,8-dihydroxy- 2-methoxy-6-methylanthraquinone) compound isolated from the sea urchins Monodictys sp. is reported to possess antimicrobial activity against the bacteria (El-Gendy et al. 2008). 2, 3 dibromabenzaldehyde-4, 5-disulphate potassium and 5-bromo-3, 4- dihydroxybenzaldehyde are isolated from Polysiphonia lanora (red alga) and is reported to have potential antibacterial activity against pathogenic bacterias (Hodgkin et al. 1966).
Polysaccharides are mainly monosaccharides sugar polymers that are linked via glycosidic/ether linkage. Polysaccharides isolated from plant sources have significant application in food and pharmaceutical industry but in the recent years many polysaccharides also have been derived from marine algae. The marine environment is being constantly explored for the extraction and isolation of novel kinds of polysaccharides. Polysaccharides such as fucoidan and laminarin are successfully used for the inhibition in growth of Staphylococcus aureus and Escherichia coli and for reducing the Helicobacter pylori biofilms in the gastric mucosa. Sulphated polysaccharides such as depolymerized fucoidans has antibacterial activity which is caused by the interaction of fucoidans with membrane proteins, leading to membrane rupture and further cell death (Fig.2). These polysaccharides are used in the food supplements to reduce the chances of Piscirickettsia salmonis infection (Hodgkin et al. 1966; Kadam et al. 2015; Yu et al. 2015; Hernández et al. 2016). Glycol-compounds showed antibacterial activity through their interaction with components of the bacterial cell wall, such as lipopolysaccharides or peptidoglycans (Besednova et al. 2015). However, in many cases, the mechanism of action is not yet understood. Sulphated polysaccharides isolated from the Sargassum swartzii are found to inhibit the growth of both gram positive and gram-negative bacteria (Vijayabaskar et al. 2012). Polysaccharides extracted from the red seaweed Pterocladia capillacea and brown seaweed Dictyopteris membranacea using hot and cold water is reported to inhibit the growth of Bacillus cereus, Staphylococcus aureus, Pseudomonas fluorescens and Escherichia coli (Abou Zeid et al. 2014).
Ulvans are a family of sulfated polysaccharides that are derived from green algae of the genus Ulva. These polymeric substances are typically found tightly linked by covalent bonds and weakly aggregated by electrostatic interactions mediated by calcium ions in the algal cell walls where they serve as structural elements (Robic et al. 2008). Ulvan is a heterogeneous sulfated polysaccharide whose composition is not univocal and significantly depends on the period of collection and the ecophysiological origin of the algal species. Ulvan structure is formally represented as the sequence of a disaccharide unit comprising two different types of aldobiuronic acid, namely ulvanobiuronic acid 3-sulfate type A (A3s) and type B (B3s) (Morelli et al. 2017). Both units are characterized by the presence of a rhamnose 3-sulfate residue linked to an uronic residue through a (1–4) glycosidic bond. The structural heterogeneity of ulvan extracted from various algal sources was primarily characterised by a different degree of polysaccharide sulfation and the substitution of uronic residues with a minor proportion of xylose, sulfated xylose, glucose, mannose, and arabinose residues (Liang et al. 2014). Ulvan, has been shown to have a wide range of biological properties and to significantly contribute to the health benefits obtained from algal food consumption. Ulvan has also been shown to anticoagulant, antioxidant, anticancer, antihyperlipidemic, antiviral, antimicrobial, and immunomodulatory properties (Morelli et al. 2017).
The amino acids having short or large peptide chains are also demonstrated to have antimicrobial potential in some case studies. Figure2 shows a schematic illustration of the main action mechanisms of antibacterial compounds extracted from different algae species. The inhibitory effects of proteins and peptides are associated with their amphiphilic nature (Lordan et al. 2011). The latter permits their interaction with polar and non-polar sites of the cell membranes. These peptides usually function by interfering the cellular processes, the interactions lead to the apparition of pores, causing disruption of the membrane and cellular rupture (Pimenta and Lebrun 2007; Nguyen et al. 2011). Protein explicitly lectin occurs in animals, human, bacteria, and algae. These lectins have wide application in human beings including carbohydrate-binding, cell adhesion, blood-protein regulation, and immune defence (Kilpatrick 2002; Ahn et al. 2007). Lectin isolated from the red algae Solieria filiformis is reported to have inhibitory action against Pseudomonas aeruginosa, Enterobacter aerogenes, Serratia marcescens, Salmonella typhi, Klebsiella pneumoniae, and Proteus sp. Lectins extracted from macroalga have gained attention owed to their great range of bioactivities. Antimicrobial peptides isolated from the algae Tetraselmis suecica (Kylin) were efficient against the gram-positive and Gram-negative bacterias (Abreu et al. 2022). An antibacterial peptides SP-1 isolated from Spirulina platensis is reported to be non-toxic by haemolysis. Furthermore, it is active against Escherichia coli and Staphylococcus aureus in the concentration range of 8mg/mL and 16mg/mL respectively (Sun et al. 2015). This SP-1 is the first antimicrobial peptide isolated from Spirulina platensis (Sun et al. 2016).
Lactones are mainly a group of cyclic esters including furanones and have been reported to possess antibacterial nature. Furanone extracts from Delisea pulchra have been used for the prevention of biofilm formation, and inhibition of quorum sensing in Pseudomonas aeruginosa. The furanones isolated from the Delisea pulchra interfere with the AHL for the LuxR receptor site that leads to virulence factor productions and pathogenesis (Brameyer and Heermann 2015). 4-bromo-5-(bromomethylene)-3-(11-hydroxybutyl)-2(5H)-furanone is isolated from the red algae Delisea pulchra which is most commonly available is reported to inhibit the production of virulence factor carbapenem during quorum sensing of Erwinia carotovora using disruption mechanism of 3-oxo-C6-HSL dependent expression of the car ABCDEFGH operon (Manefield et al. 2001). Researchers have also reported a combination of some phytochemicals isolated from plant source against the common food poisoning causing bacteria like Campylobacter jejuni responsible for causing the camphylobacteriosis (Altekruse et al. 1999). The isolated furan from Delisea pulchra combined with the epigallocatechin gallate from green tea and citrus acid extract leads to significant decrease in the AI-2 activity, bacterial motility, and biofilm formation in Campylobacter jejuni (Castillo et al. 2015). Algal furanones can also be exploited in place of synthetic sanitisers and antibiotics. These algal furanones have also demonstrated its activity against the Pseudomonas aeruginosa infection which is responsible for causing the mucoid films in the lungs of patients suffering from the problem of cystic fibrosis (Chatterjee et al. 2016). Antibacterial activity of algal lipids and fatty acids has been attributed to their ability to inhibit the electron transport chain and oxidative phosphorylation in cell membranes, leading to the formation of peroxidation and auto-oxidation degradation products and the cellular lysis (Fig.2). To the best of our knowledge, no studies have first isolated and then confirmed the antibacterial activity of macro algal fatty acids. The list of antibacterial activity of some important algae is given in Table2 (Desbois and Smith 2010).
Antifungal bioactive from algae
Marine algae are not only the source of antibacterial compounds but also a large source of antifungal compounds. Fungal infections are common diseases but sometimes these become dangerous due to the development of antibiotic resistance. At present many pharmaceutical firms have developed many broad-spectrum antifungal antibiotics for the treatment and prevention of such kind of infections but these are evolving resistance amongst them. Besides using natural drugs isolated from plant sources for the treatment of such kinds of diseases scientists are shifting towards the use of algae from the marine environment. As a lot of research is being conducted in the field of ethno-pharmacology for the isolation of active drugs for the treatment and cure. However, the concern related to its endangered status has limited the use of these. The utilization of marine algae is also considered as the alternative for it. This section focuses on the antifungals derived from the marine algae, and organic algal extracts used as antifungals, their mechanism of actions, as well as potential applications as antibiotics etc. Figure2 describes the main action mechanisms of antifungal compounds extracted from different algae species, such as the antifungal properties of algae polysaccharides are accredited to the contact of glyco-receptors of the bacterial cell wall, compounds of the membrane and nucleic acids and the polysaccharides. Laurencia composita is a common red alga and four new antifungal compounds laurecomins A-D were isolated from it. Among the isolated compounds laurecomin B is reported to have antifungal activity against the Colletotrichum lagenarium (formed ~ 10mm zone of inhibition) (Li et al. 2012; Liang et al. 2014). Several new brominated sesquiterpenes, seco-laurokamurone, laurepoxyene, 3β-hydroperoxyaplysin, 3α-hydroperoxy-3-epiaplysin, 8,10-dibromoisoaplysin, and laurokamurene D were isolated from the Laurencia okamurai (red algae). Simultaneously, its antifungal activity is reported against the Cryptococcus neoformans, Candida glabrata, Trichophyton rubrum and Aspergillus fumigatus (Yu et al. 2015). Symphyocladia latiuscula a member of red algae is reported to have rich source of brominated phenols. From these various antifungals were reported including symphyocladin A, symphyocladin B, symphyocladin C, symphyocladin D, symphyocladin E, symphyocladin F, symphyocladin G and Bromocatechols. Amid these isolated compounds only Bromocatechols showed antifungal potential against the fungi Candida albicans (Xu et al. 2012, 2013; Xu et al. 2014). (Cantrell et al. 2005) reported presence of four antifungal components from the Haplophyllum sieversii as bioactive alkaloids flindersine, anhydroevoxine, haplamine, and a lignan eudesmin. Out of these alkaloids flindersine exhibited most effective bioactivity against C. fragariae, C. gloeosporioides, C. acutatum, Botrytis cinerea, Fusarium oxysporum, and Phomopsis obscurans. Out of these Haplamine demonstrated selective inhibition against the odor-producing cyanobacterium O. perornata compared to the activity against the green alga S. capricornutum. Dieckol is an antifungal activity producing agent extracted from marine brown alga. Ecklonia cava is reported to have potent activity against common pathogen Trichophyton rubrum. The MIC of dieckol produced against Trichophyton rubrum was reported to be 200 µM. The antifungal activity was chiefly due to disruption and perforation of cytoplasmic membrane integrity caused by dieckol (Lee 2010). Similarly, Methoxybifurcarenone an antifungal meroditerpenoid is derived from the brown alga Cystoseira tamariscifolia. Researchers have reported its activity against the common tomato fungal pathogen Botrytis cinerea, Fusarium oxysporum sp. mycopersici and Verticillium alboatrum (Bennamara et al. 1999; Besednova et al. 2015). Fucofuroeckol-A isolated from the edible alga Eisenia bicyclis is reported to have antifungal activity against the fluconazole-resistant Candida albicans at the MIC of 512µg mL-1 (Kim et al. 2018). Lectins extracted from algae species exhibited antifungal activity. Interactions between these molecules and membranes lead to the disruption of the membrane stability and cellular functions (Fig.2). Numerous factors can affect this activity viz., the molecular weight, charge density, structure, and conformation (Singh and Walia 2018; Barre et al. 2019).
The antifungal agents derived from marine algae are relatively less reported and active research in isolating such components are rather slow. However, many marine algae solvent extracts have also been publicized for their antifungal activity against pathogenic fungus causing serious illness in animals as well as human beings. In the case of fungi, it has also been proposed that fatty acids may act in disrupting the cell membrane, inhibiting the reproduction. Some polar and non-polar extracts of Colpomenia sinuosa, Padina pavonia, Cystoseira barbata and Sargassum vulgare were tested for their antifungal activity against the Aspergillus niger, (A) flavus, Penicillium parasiticus, Candida utilis and Fusarium solani among which methanolic extract of C. barbata showed the best activity. The active constituents which were found to be quite effective through GC-MS analysis were indoles, terpenes, acetogenins, phenols, and volatile halogenated hydrocarbons (Boughalleb et al. 2009). The antifungal activity of Bifurcaria bifurcate a common seaweed of order Fucales (Ochrophyta, Phaeophyceae) that is available all around the year reveals its maiden antifungal activity against some dermatophytes. The methanolic extract of (B) bifurcate was found to be most effective against the E. floccosum (Carvalho et al. 2019). The antifungal activity of six species of marine macro-algae i.e. Codium decorticatum, Caulerpa scalpelliformis, Gracilaria crassa, Acanthophora spicifera, Sargassum wightii and Turbinaria conoides using different solvents (i.e. acetone, methanol, chloroform, diethyl ether, ethyl acetate, and hexane) were evaluated against Fusarium oxysporum, F. udum, F. solani, Rhizoctonia solani, Alternaria alternat, Botrytis cinerea, Candida albicans, Candida krusei, Aspergillus niger and Aspergillus flavus. The results of this work testified that, the maximum activity was reported from the families of Phaeophyceae, Chlorophyceae and Rhodophyceae, respectively. Nevertheless, the maximum activity was reported by using the acetone extract of Turbinaria conoides against F. udum. This result showed that brown seaweed T. conoides is found to be most effective as compared to green and red seaweeds (Lavanya and Veerappan 2012) Fucoidan may not present a direct killing effect and may act by trapping nutrients, reducing the bioavailability. To our knowledge, few studies have evaluated the antifungal properties and mechanisms of algal polysaccharides. The summary of these algal phytochemicals are given in the Table3.
Anticoagulant and immunosuppressive applications from algae
The anticoagulant action is measured by the extension of activated partial thromboplastin time, thrombin time, and prothrombin time (Morelli et al. 2017). According to the findings, these substances have antiplatelet, anticoagulant proteins with fibrinolytic enzymes that can alter endothelial cell activities and activate the fibrinolysis system. Algae-derived phlorotannins and SPs have shown considerable promise in global health as anticoagulant drugs. Immunosuppressive substances can suppress the immune system, particularly T and B cells, by various ways (Boughalleb et al. 2009; Kim and Wijesekara 2011). They are required to increase the survival of allogeneic organ transplants by reducing host immune responses. Fucoidan and laminarans from brown algae, carrageenan from red algae, and ulvan from green algae are the principal SPs that have received a lot of interest in the food, cosmetic, and pharmaceutical industries (Usman et al. 2017). Some natural and safe sources of anticoagulant agents generating phenolic compound are Ecklonia cava, E. stolonifera, E. kurome, Eisenia bicyclis, Ishige okamurae, S. thunbergii, and Hizikia fusiformis. Sulfolipids from blue-green algae has shown strong immunosuppressive effect in human-mixed lymphocyte reaction, which does not affect the general immunocompetence (Kim and Wijesekara 2011). Spirulina can modulate the production of cytokines by human peripheral blood mononuclear cells. Therapeutic use of Spirulina has been explored, by reducing the levels of glucose and lipids serum, protects the kidney against heavy metals and drugs. β-1,3 glucan from Chlorella sp.reduces free radicals and blood cholesterol (Kim and Wijesekara 2011).
Antiviral applications of algae metabolites
Despite earlier vaccine developments providing acquired immunity, current efforts to develop antiviral drugs have increased dramatically as a result of the emergence or (re)emergence of contagious diseases. Drug resistance may develop as a result of viruses’ ability to change their genetic composition autonomously in response to each interaction with a treatment strategy. For the last three years the entire world has faced the challenges of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2). The SARS-CoV-2 has been identified as a single-stranded, positive-sense RNA virus belonging to the Beta coronavirus family. The disease was first diagnosed at the end of 2019 in Wuhan and later on WHO proclaimed that the illness brought on by this virus was a global pandemic. Researchers incessantly tried to develop and implement rapid diagnoses, safe and effective vaccinations and other alternative therapeutic procedures to combat this deadly disease (Lai et al. 2020; Kumar et al. 2022). However, synthetic drug caused side effects and high costs to benefit ratio changed researcher’s interests in natural product-based medicines. Algae are reported to be a rich source of antiviral compounds with immunity-boosting properties such as lectins and sulphated polysaccharides. Therefore, antiviral substances derived from natural resources and some medicines have seen a boom in popularity recently. Algal-derived metabolites can be also used as antibodies and vaccine raw materials against COVID-19 (Kumar et al. 2022). Recent studies have reported that these compounds demonstrate substantial activity against a wide array of DNA and RNA viruses, including the influenza virus known to be associated with respiratory illnesses (Ben Hlima et al. 2022). However, this field of study is still in its early stages of development but we are certain that in future algal species will be used as immunity boosters to reduce viral activity in humans and be recommended for usage as a COVID-19 preventative measure too. Carrageenan from the red algae Gigartina skottsbergii, for example, has been found to bind enveloped and non-enveloped viruses. The SP stops viruses like dengue virus (DENV), human papillomaviruses (HPVs), and HIV from attaching to host cells and internalising them. Furthermore, Galactan, alginate, and fucan from red algae, laminarin from brown algae, naviculan from Diatom Navicula directa, calcium spirulan, and nostaflan from blue-green algae have been discovered to have remarkable antiviral properties against HIV, HPV, DENV, and HSV. Table4 provides a comprehensive review of the literature for antiviral activity of different algal strains with their active metabolite and indicates that species belonging to rhodophyceae, phaeophyceae and chlorophyceae are potent against viral infections.
Conclusion and future prospective
Modern antibiotics brought a new age of medicine. Currently, society is facing a major threat to the health of the populations as some bacteria have developed resistance to contemporary antibiotics. To solve this problem alga and their products are frequently being used in numerous countries. As marine algae are a gigantic source of antimicrobials having activity of both bactericidal as well as antifungal in nature. Many essential phytochemicals are also isolated from these which have wide range of activity. Presently, least exposure is provided for the exploration of antimicrobial compounds from these naturally abundant resources and hence more attention is required for isolation of active components from these. Algae are known to produce various kinds of natural products with potential antimicrobial activity against the pathogenic microbes. As demonstrated by this review, algae bio-products such as proteins, peptides, polysaccharides, polyphenols, fatty acids, and pigments are among the most valuable antimicrobial compounds. Furthermore, Algal derived secondary metabolites with high medicinal value have been isolated and supplemented with active pharmacological ingredients for anticancer properties. Cyanobacteria viz. Nostoc, Spirulina, and Oscillatoria produce cytotoxic lipopeptides (lyngbyabellins, didemnin, and hectochlorin) via a combination of anabolic fatty synthesis and acetyl Co-A, have shown to trigger caspase-8-dependent apoptotic pathway and induce tumour suppression in various cancer cell lines that include melanoma, leukaemia, carcinoma, myeloma, and neuroblastoma types. Borophycin, a boron-containing metabolite produced by N. spongiaeforme var. tenue, has a potent cytotoxic effect in human carcinoma. Apratoxin, microcystins, cryptophycins, anatoxin-A, and many other peptide toxins, a natural metabolite derived from cyanobacteria have demonstrated clinical efficacy for different types of cancers. For the futuristic applications algae should be exploited for the isolation of antiviral agents against the current havoc of Covid-19 corona pathogens causing the pandemic. Further it is also required to develop algae as the therapeutic agents in the form of food that can be consumed in daily diet. This would need further investigations for the identification of potential algal species, standardization of analytical methods, isolation of compounds through bioassay guided fractionation, detailed chemical characterization and evaluation of their safety. Simultaneously, evaluation of synergistic effects among the components, and efforts to enhance the yields and lower the extraction costs are needed. Further work in the development of new and better large-scale algal culture systems is also required.
Data availability
Not applicable.
Code availability
Not applicable.
References
Abdelmohsen UR, Balasubramanian S, Oelschlaeger TA, Grkovic T, Pham NB, Quinn RJ, Hentschel U (2017) Potential of marine natural products against drug-resistant fungal, viral, and parasitic infections. Lancet Infect Dis 17:e30–e41. https://doi.org/10.1016/S1473-3099(16)30323-1
Abou Zeid AH, Aboutabl EA, Sleem AA, El-Rafie HM (2014) Water soluble polysaccharides extracted from Pterocladia capillacea and Dictyopteris membranacea and their biological activities. Carbohydr Polym 113:62–66. https://doi.org/10.1016/j.carbpol.2014.06.004
Abreu TM, Corpe FP, Teles FB, da Conceição Rivanor RL, de Sousa CNS, da Silva Medeiros I, de Queiroz INL, Figueira-Mansur J, Mota ÉF, Mohana-Borges R, Macedo DS, de Vasconcelos SMM, Júnior JERH, Benevides NMB (2022) Lectin isolated from the red marine alga Solieria filiformis (Kützing) P.W. Gabrielson: Secondary structure and antidepressant-like effect in mice submitted to the lipopolysaccharide-induced inflammatory model of depression. Algal Res 65:102715. https://doi.org/10.1016/j.algal.2022.102715
Ahmed N, Mohamed HF, Xu C, Sun X, Huang L (2022) Novel antibacterial activity of Sargassum fusiforme extract against coral white band disease. Electron J Biotechnol 57:12–23. https://doi.org/10.1016/j.ejbt.2022.03.002
Ahn G-N, Kim K-N, Cha S-H, Song C-B, Lee J, Heo M-S, Yeo I-K, Lee N-H, Jee Y-H, Kim J-S, Heu M-S, Jeon Y-J (2007) Antioxidant activities of phlorotannins purified from Ecklonia cava on free radical scavenging using ESR and H2O2-mediated DNA damage. Eur Food Res Technol 226:71–79. https://doi.org/10.1007/s00217-006-0510-y
Algotiml R, Gab-Alla A, Seoudi R, Abulreesh HH, El-Readi MZ, Elbanna K (2022) Anticancer and antimicrobial activity of biosynthesized Red Sea marine algal silver nanoparticles. Sci Rep 12:2421. https://doi.org/10.1038/s41598-022-06412-3
Alsenani F, Tupally KR, Chua ET, Eltanahy E, Alsufyani H, Parekh HS, Schenk PM (2020) Evaluation of microalgae and cyanobacteria as potential sources of antimicrobial compounds. Saudi Pharm J 28:1834–1841. https://doi.org/10.1016/j.jsps.2020.11.010
Altekruse SF, Stern NJ, Fields PI, Swerdlow DL (1999) Campylobacter jejuni- An Emerging Foodborne Pathogen. Emerg Infect Dis 5:28–35. https://doi.org/10.3201/eid0501.990104
Ancheeva E, El-Neketi M, Daletos G, Ebrahim W, Song W, Lin W, Proksch P (2018) Anti-infective Compounds from Marine Organisms. In: Rampelotto PH, Trincone A (eds) Grand Challenges in Marine Biotechnology. Springer International Publishing, Cham, pp 97–155. https://doi.org/10.1007/978-3-319-69075-9_3
Barre A, Simplicien M, Benoist H, Van Damme EJM, Rougé P (2019) Mannose-Specific Lectins from Marine Algae: Diverse Structural Scaffolds Associated to Common Virucidal and Anti-Cancer Properties. Mar Drugs 17:440. https://doi.org/10.3390/md17080440
Beaulieu L, Bondu S, Doiron K, Rioux L-E, Turgeon SL (2015) Characterization of antibacterial activity from protein hydrolysates of the macroalga Saccharina longicruris and identification of peptides implied in bioactivity. J Funct Foods 17:685–697. https://doi.org/10.1016/j.jff.2015.06.026
Ben Hlima H, Farhat A, Akermi S, Khemakhem B, Ben Halima Y, Michaud P, Fendri I, Abdelkafi S (2022) In silico evidence of antiviral activity against SARS-CoV-2 main protease of oligosaccharides from Porphyridium sp. Sci Total Environ 836:155580. https://doi.org/10.1016/j.scitotenv.2022.155580
Bennamara A, Abourriche A, Berrada M, Charrouf M, Chaib N, Boudouma M, Garneau FX (1999) Methoxybifurcarenone: an antifungal and antibacterial meroditerpenoid from the brown alga Cystoseira tamariscifolia. Phytochemistry 52:37–40. https://doi.org/10.1016/S0031-9422(99)00040-0
Besednova NN, Zaporozhets TS, Somova LM, Kuznetsova TA (2015) Review: prospects for the use of extracts and polysaccharides from marine algae to prevent and treat the diseases caused by Helicobacter pylori. Helicobacter 20:89–97. https://doi.org/10.1111/hel.12177
Bhalodia NR, Shukla VJ (2011) Antibacterial and antifungal activities from leaf extracts of Cassia fistula l.: An ethnomedicinal plant. J Adv Pharm Technol Res 2:104–109. https://doi.org/10.4103/2231-4040.82956
Blunt J, Buckingham J, Munro M (2012) Taxonomy and Marine Natural Products Research. In: Fattorusso E, Gerwick WH, Taglialatela-Scafati O (eds) Handbook of Marine Natural Products. Springer Netherlands, Dordrecht, pp 3–54. https://doi.org/10.1007/978-90-481-3834-0_1
Bogolitsyn K, Dobrodeeva L, Druzhinina A, Ovchinnikov D, Parshina A, Shulgina E (2019) Biological activity of a polyphenolic complex of Arctic brown algae. J Appl Phycol 31:3341–3348. https://doi.org/10.1007/s10811-019-01840-7
Boughalleb N, Trabelsi L, Harzallah-Skhiri F (2009) Antifungal activity from polar and non-polar extracts of some Chenopodiaceae wild species growing in Tunisia. Nat Prod Res 23:988–997. https://doi.org/10.1080/14786410802168494
Bouhlal R, Haslin C, Chermann J-C, Colliec-Jouault S, Sinquin C, Simon G, Cerantola S, Riadi H, Bourgougnon N (2011) Antiviral activities of sulfated polysaccharides isolated from Sphaerococcus coronopifolius (Rhodophytha, Gigartinales) and Boergeseniella thuyoides (Rhodophyta, Ceramiales). Mar Drugs 9:1187–1209. https://doi.org/10.3390/md9071187
Brameyer S, Heermann R (2015) Specificity of Signal-Binding via Non-AHL LuxR-Type Receptors. PLoS ONE 10:e0124093. https://doi.org/10.1371/journal.pone.0124093
Cabral EM, Mondala JRM, Oliveira M, Przyborska J, Fitzpatrick S, Rai DK, Sivagnanam SP, Garcia-Vaquero M, O’Shea D, Devereux M, Tiwari BK, Curtin J (2021) Influence of molecular weight fractionation on the antimicrobial and anticancer properties of a fucoidan rich-extract from the macroalgae Fucus vesiculosus. Int J Biol Macromol 186:994–1002. https://doi.org/10.1016/j.ijbiomac.2021.06.182
Cakmak YS, Kaya M, Asan-Ozusaglam M (2014) Biochemical composition and bioactivity screening of various extracts from Dunaliella salina, a green microalga. EXCLI J 13:679–690 PMID: 26417292
Cantrell CL, Schrader KK, Mamonov LK, Sitpaeva GT, Kustova TS, Dunbar C, Wedge DE (2005) Isolation and Identification of Antifungal and Antialgal Alkaloids from Haplophyllum sieversii. J Agric Food Chem 53:7741–7748. https://doi.org/10.1021/jf051478v
Carvalho GL, Silva R, Gonçalves JM, Batista TM, Pereira L (2019) Extracts of the seaweed Bifurcaria bifurcata display antifungal activity against human dermatophyte fungi. J Ocean Limnol 37:848–854. https://doi.org/10.1007/s00343-019-8118-9
Castillo S, Heredia N, García S (2015) 2(5H)-Furanone, epigallocatechin gallate, and a citric-based disinfectant disturb quorum-sensing activity and reduce motility and biofilm formation of Campylobacter jejuni. Folia Microbiol 60:89–95. https://doi.org/10.1007/s12223-014-0344-0
Chatterjee M, Anju CP, Biswas L, Anil Kumar V, Gopi Mohan C, Biswas R (2016) Antibiotic resistance in Pseudomonas aeruginosa and alternative therapeutic options. Int J Med Microbiol 306:48–58. https://doi.org/10.1016/j.ijmm.2015.11.004
Cherian C, Jannet Vennila J, Sharan L (2019) Marine bromophenols as an effective inhibitor of virulent proteins (peptidyl arginine deiminase, gingipain R and hemagglutinin A) in Porphyromas gingivalis. Archives of Oral Biology 100:119–128. https://doi.org/10.1016/j.archoralbio.2019.02.016
Chong CW, Hii SL, Wong CL (2011) Antibacterial activity of Sargassum polycystum C. Agardh and Padina australis Hauck (Phaeophyceae). Afr J Biotechnol 10:14125–14131. https://doi.org/10.5897/AJB11.966
Christabell J, Lipton AP, Aishwarya MS, Sarika AR, Udayakumar A (2011) Antibacterial activity of aqueous extract from selected macroalgae of southwest coast of India. Seaweed Res Utilization 33:67–75
Cui J, Ren B, Tong Y, Dai H, Zhang L (2015) Synergistic combinations of antifungals and anti-virulence agents to fight against Candida albicans. Virulence 6:362–371. https://doi.org/10.1080/21505594.2015.1039885
Cusson AJ, Burkholder KM, Byron CJ, Grebe GS, Deveau AM (2021) Impact of time of harvest and drying method on antimicrobial activity of Saccharina latissima against two Staphylococcus aureus strains. Appl Phycol 2:80–88. https://doi.org/10.1080/26388081.2021.1996208
de Borba MC, Velho AC, de Freitas MB, Holvoet M, Maia-Grondard A, Baltenweck R, Magnin-Robert M, Randoux B, Hilbert J-L, Reignault P, Hugueney P, Siah A, Stadnik MJ (2022) A Laminarin-Based Formulation Protects Wheat Against Zymoseptoria tritici via Direct Antifungal Activity and Elicitation of Host Defense-Related Genes. Plant Dis 106:1408–1418. https://doi.org/10.1094/pdis-08-21-1675-re
de Felício R, de Albuquerque S, Young MCM, Yokoya NS, Debonsi HM (2010) Trypanocidal, leishmanicidal and antifungal potential from marine red alga Bostrychia tenella J. Agardh (Rhodomelaceae, Ceramiales). J Pharm Biomed Anal 52:763–769. https://doi.org/10.1016/j.jpba.2010.02.018
Desbois AP, Smith VJ (2010) Antibacterial free fatty acids: activities, mechanisms of action and biotechnological potential. Appl Microbiol Biotechnol 85:1629–1642. https://doi.org/10.1007/s00253-009-2355-3
El Shafay SM, Ali SS, El-Sheekh MM (2016) Antimicrobial activity of some seaweeds species from Red sea, against multidrug resistant bacteria. Egypt J Aquat Res 42:65–74. https://doi.org/10.1016/j.ejar.2015.11.006
El-Gendy MMA, Hawas UW, Jaspars M (2008) Novel Bioactive Metabolites from a Marine Derived Bacterium Nocardia sp. ALAA 2000. J Antibiot 61:379–386. https://doi.org/10.1038/ja.2008.53
Eom S-H, Lee D-S, Jung Y-J, Park J-H, Choi J-I, Yim M-J, Jeon J-M, Kim H-W, Son K-T, Je J-Y, Lee M-S, Kim Y-M (2014) The mechanism of antibacterial activity of phlorofucofuroeckol-A against methicillin-resistant Staphylococcus aureus. Appl Microbiol Biotechnol 98:9795–9804. https://doi.org/10.1007/s00253-014-6041-8
Fabricant DS, Farnsworth NR (2001) The value of plants used in traditional medicine for drug discovery. Environ Health Perspect 109:69–75. https://doi.org/10.1289/ehp.01109s169
Fang H-Y, Chiou S-F, Uvarani C, Wen Z-H, Hsu C-H, Wu Y-C, Wang W-L, Liaw C-C, Sheu J-H (2014a) Cytotoxic, Anti-inflammatory, and Antibacterial Sulfur-Containing Polybromoindoles from the Formosan Red Alga Laurencia brongniartii. BCSJ 87:1278–1280. https://doi.org/10.1246/bcsj.2014a0165
Fang H-Y, Chiou S-F, Uvarani C, Wen Z-H, Hsu C-H, Wu Y-C, Wang W-L, Liaw C-C, Sheu J-H (2014b) Cytotoxic, Anti-inflammatory, and Antibacterial Sulfur-Containing Polybromoindoles from the Formosan Red Alga Laurencia brongniartii. BCSJ 87:1278–1280. https://doi.org/10.1246/bcsj.2014b0165
Faulkner DJ (1998) Marine natural products. Nat Prod Rep 15:113–158. https://doi.org/10.1039/D2NP00021K
Fayzi L, Askarne L, Boufous EH, Cherifi O, Cherifi K (2022) Antioxidant and Antifungal Activity of Some Moroccan Seaweeds Against Three Postharvest Fungal Pathogens. Asian J of Plant Sciences 21:328–338. https://doi.org/10.3923/ajps.2022.328.338
Fitchett JR (2015) Antibiotics, copayments, and antimicrobial resistance: investment matters. Lancet Infect Dis 15:1125–1127. https://doi.org/10.1016/S1473-3099(15)00057-2
França PHB, Barbosa DP, da Silva DL, Ribeiro ÊAN, Santana AEG, Santos BVO, Barbosa-Filho JM, Quintans JSS, Barreto RSS, Quintans-Júnior LJ, de Araújo-Júnior JX (2014) Indole Alkaloids from Marine Sources as Potential Leads against Infectious Diseases. Biomed Res Int. 2014: 375423. https://doi.org/10.1155/2014/375423
Frazzini S, Scaglia E, Dell’Anno M, Reggi S, Panseri S, Giromini C, Lanzoni D, Sgoifo Rossi CA, Rossi L (2022) Antioxidant and Antimicrobial Activity of Algal and Cyanobacterial Extracts: An In Vitro Study. Antioxidants 11:992. https://doi.org/10.3390/antiox11050992
Geetha Bai R, Tuvikene R (2021) Potential Antiviral Properties of Industrially Important Marine Algal Polysaccharides and Their Significance in Fighting a Future Viral Pandemic. Viruses 13:1817. https://doi.org/10.3390/v13091817
Greff S, Zubia M, Genta-Jouve G, Massi L, Perez T, Thomas OP (2014) Mahorones, Highly Brominated Cyclopentenones from the Red Alga Asparagopsis taxiformis. J Nat Prod 77:1150–1155. https://doi.org/10.1021/np401094h
Gribble G (2015) Biological Activity of Recently Discovered Halogenated Marine Natural Products. Mar Drugs 13:4044–4136. https://doi.org/10.3390/md13074044
Hernández AJ, Romero A, Gonzalez-Stegmaier R, Dantagnan P (2016) The effects of supplemented diets with a phytopharmaceutical preparation from herbal and macroalgal origin on disease resistance in rainbow trout against Piscirickettsia salmonis. Aquaculture 454:109–117. https://doi.org/10.1016/j.aquaculture.2015.12.016
Hodgkin JH, Craigie JS, McInnes AG (1966) The occurrence of 2,3-dibromobenzyl alcohol 4,5-disulfate, dipotassium salt, in polysiphonia lanosa. Can J Chem 44:74–78. https://doi.org/10.1139/v66-012
Ismail A, Ktari L, Redjem Romdhane B, Aoun Y, Sadok B, Boudabous S, El Bour M (2018) Antimicrobial Fatty Acids from Green Alga Ulva rigida (Chlorophyta). Biomed Res Int 2018:1–12. https://doi.org/10.1155/2018/3069595
Jun J-Y, Jung M-J, Jeong I-H, Yamazaki K, Kawai Y, Kim B-M (2018) Antimicrobial and Antibiofilm Activities of Sulfated Polysaccharides from Marine Algae against Dental Plaque Bacteria. Mar Drugs 16:301. https://doi.org/10.3390/md16090301
Kadam S, O’Donnell C, Rai D, Hossain M, Burgess C, Walsh D, Tiwari B (2015) Laminarin from Irish Brown Seaweeds Ascophyllum nodosum and Laminaria hyperborea: Ultrasound Assisted Extraction, Characterization and Bioactivity. Mar Drugs 13:4270–4280. https://doi.org/10.3390/md13074270
Kamei Y, Sueyoshi M, Hayashi K, Terada R, Nozaki H (2009) The novel anti-Propionibacterium acnes compound, Sargafuran, found in the marine brown alga Sargassum macrocarpum. J Antibiot 62:259–263. https://doi.org/10.1038/ja.2009.25
Kilpatrick D (2002) Animal lectins: a historical introduction and overview. Biochimica et Biophysica Acta (BBA) -. Gen Subj 1572:187–197. https://doi.org/10.1016/S0304-4165(02)00308-2
Kim K-H, Yu D, Eom S-H, Kim H-J, Kim D-H, Song H-S, Kim D-M, Kim Y-M (2018) Fucofuroeckol-A from edible marine alga Eisenia bicyclis to restore antifungal activity of fluconazole against fluconazole-resistant Candida albicans. J Appl Phycol 30:605–609. https://doi.org/10.1007/s10811-017-1232-1
Kim S-K, Wijesekara I (2011) Anticoagulant Effect of Marine Algae. pp.235–244. In: Kim S.-K. (eds), Advances in Food and Nutrition Research, Academic Press. https://doi.org/10.1016/B978-0-12-387669-0.00018-1
Kosanić M, Ranković B, Stanojković T (2019) Brown macroalgae from the Adriatic Sea as a promising source of bioactive nutrients. Food Measure 13:330–338. https://doi.org/10.1007/s11694-018-9948-4
Kumar A, Singh RP, Kumar I, Yadav P, Singh SK, Kaushalendra, Singh PK, Gupta RK, Singh SM, Kesawat MS, Saratale GD, Chung S-M, Kumar M (2022) Algal Metabolites Can Be an Immune Booster against COVID-19 Pandemic. Antioxidants 11:452. https://doi.org/10.3390/antiox11030452
Kurhekar JV (2020) Antimicrobial lead compounds from marine plants. pp.257–274. Phytochemicals as Lead Compounds for New Drug Discovery, Elsevier. https://doi.org/10.1016/B978-0-12-817890-4.00017-2
Lai C-C, Shih T-P, Ko W-C, Tang H-J, Hsueh P-R (2020) Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): The epidemic and the challenges. Int J Antimicrob Agents 55:105924. https://doi.org/10.1016/j.ijantimicag.2020.105924
Lavanya R, Veerappan N (2012) Pharmaceutical Properties of Marine Macroalgal Communities from Gulf of Mannar against Human Fungal Pathogens. Asian Pac J Trop Disease 2:S320–S323. https://doi.org/10.1016/S2222-1808(12)60174-1
Lee J-B, Hayashi K, Hashimoto M, Nakano T, Hayashi T (2004) Novel antiviral fucoidan from sporophyll of Undaria pinnatifida (Mekabu). Chem Pharm Bull (Tokyo) 52:1091–1094. https://doi.org/10.1248/cpb.52.1091
Lee MH (2010) Antifungal Activities of Dieckol Isolated from the Marine Brown Alga Ecklonia cava against Trichophyton rubrum. J Korean Soc Appl Biol Chem 53:504–507. https://doi.org/10.3839/jksabc.2010.076
Levy SB, Marshall B (2004) Antibacterial resistance worldwide: causes, challenges and responses. Nat Med 10:S122–S129. https://doi.org/10.1038/nm1145
Li X-D, Miao F-P, Li K, Ji N-Y (2012) Sesquiterpenes and acetogenins from the marine red alga Laurencia okamurai. Fitoterapia 83:518–522. https://doi.org/10.1016/j.fitote.2011.12.018
Liang W, Mao X, Peng X, Tang S (2014) Effects of sulfate group in red seaweed polysaccharides on anticoagulant activity and cytotoxicity. Carbohydr Polym 101:776–785. https://doi.org/10.1016/j.carbpol.2013.10.010
Liu M, Liu Y, Cao M-J, Liu G-M, Chen Q, Sun L, Chen H (2017) Antibacterial activity and mechanisms of depolymerized fucoidans isolated from Laminaria japonica. Carbohydr Polym 172:294–305. https://doi.org/10.1016/j.carbpol.2017.05.060
Lopes G, Pinto E, Andrade PB, Valentão P (2013) Antifungal Activity of Phlorotannins against Dermatophytes and Yeasts: Approaches to the Mechanism of Action and Influence on Candida albicans Virulence Factor. PLoS ONE 8:e72203. https://doi.org/10.1371/journal.pone.0072203
Lordan S, Ross RP, Stanton C (2011) Marine Bioactives as Functional Food Ingredients: Potential to Reduce the Incidence of Chronic Diseases. Mar Drugs 9:1056–1100. https://doi.org/10.3390/md9061056
Manefield M, Welch M, Givskov M, Salmond GPC, Kjelleberg S (2001) Halogenated furanones from the red alga, Delisea pulchra, inhibit carbapenem antibiotic synthesis and exoenzyme virulence factor production in the phytopathogen Erwinia carotovora. FEMS Microbiol Lett 205:131–138. https://doi.org/10.1016/S0378-1097(01)00460-8
Martín-Martín RP, Carcedo-Forés M, Camacho-Bolós P, García-Aljaro C, Angulo-Preckler C, Avila C, Lluch JR, Garreta AG (2022) Experimental evidence of antimicrobial activity in Antarctic seaweeds: ecological role and antibiotic potential. Polar Biol 45:923–936. https://doi.org/10.1007/s00300-022-03036-1
McRae J, Yang Q, Crawford R, Palombo E (2007) Review of the methods used for isolating pharmaceutical lead compounds from traditional medicinal plants. Environmentalist 27:165–174. https://doi.org/10.1007/s10669-007-9024-9
Morán-Santibañez K, Peña-Hernández M, Cruz-Suárez L, Ricque-Marie D, Skouta R, Vasquez A, Rodríguez-Padilla C, Trejo-Avila L (2018) Virucidal and Synergistic Activity of Polyphenol-Rich Extracts of Seaweeds against Measles Virus. Viruses 10:465. https://doi.org/10.3390/v10090465
Morelli A, Puppi D, Chiellini F (2017) Perspectives on Biomedical Applications of Ulvan. Seaweed Polysaccharides, pp 305–330. https://doi.org/10.1016/B978-0-12-809816-5.00016-5
Moubayed NMS, Al Houri HJ, Al Khulaifi MM, Al Farraj DA (2017) Antimicrobial, antioxidant properties and chemical composition of seaweeds collected from Saudi Arabia (Red Sea and Arabian Gulf). Saudi J Biol Sci 24:162–169. https://doi.org/10.1016/j.sjbs.2016.05.018
Nguyen LT, Haney EF, Vogel HJ (2011) The expanding scope of antimicrobial peptide structures and their modes of action. Trends in Biotechnology 29:464–472. https://doi.org/10.1016/j.tibtech.2011.05.001
Pérez MJ, Falqué E, Domínguez H (2016) Antimicrobial Action of Compounds from Marine Seaweed. Mar Drugs 14:E52. https://doi.org/10.3390/md14030052
Pimenta DC, Lebrun I (2007) Cryptides: Buried secrets in proteins. Peptides 28:2403–2410. https://doi.org/10.1016/j.peptides.2007.10.005
Pina-Pérez MC, Rivas A, Martínez A, Rodrigo D (2017) Antimicrobial potential of macro and microalgae against pathogenic and spoilage microorganisms in food. Food Chem 235:34–44. https://doi.org/10.1016/j.foodchem.2017.05.033
Pradhan B, Patra S, Dash SR, Satapathy Y, Nayak S, Mandal AK, Jena M (2022) In vitro antidiabetic, anti-inflammatory and antibacterial activity of marine alga Enteromorpha compressa collected from Chilika lagoon, Odisha, India. https://doi.org/10.1007/s42535-022-00359-6. Vegetos
Prakash JW, Antonisamy JM, Jeeva S (2011) Antimicrobial activity of certain fresh water microalgae from Thamirabarani River, Tamil Nadu, South India. Asian Pac J Trop Biomed 1:S170–S173. https://doi.org/10.1016/S2221-1691(11)60149-4
Rahelivao M, Gruner M, Andriamanantoanina H, Andriamihaja B, Bauer I, Knölker H-J (2015) Red Algae (Rhodophyta) from the Coast of Madagascar: Preliminary Bioactivity Studies and Isolation of Natural Products. Mar Drugs 13:4197–4216. https://doi.org/10.3390/md13074197
Rajauria G, Abu-Ghannam N (2013) Isolation and Partial Characterization of Bioactive Fucoxanthin from Himanthalia elongata Brown Seaweed: A TLC-Based Approach. International Journal of Analytical Chemistry. 2013: 1–6
Robic A, Sassi J-F, Lahaye M (2008) Impact of stabilization treatments of the green seaweed Ulva rotundata (Chlorophyta) on the extraction yield, the physico-chemical and rheological properties of ulvan. Carbohydr Polym 74:344–352. https://doi.org/10.1016/j.carbpol.2008.02.020
Ryu YB, Jeong HJ, Yoon SY, Park J-Y, Kim YM, Park S-J, Rho M-C, Kim S-J, Lee WS (2011) Influenza Virus Neuraminidase Inhibitory Activity of Phlorotannins from the Edible Brown Alga Ecklonia cava. J Agric Food Chem 59:6467–6473. https://doi.org/10.1155/2013/802573
Siahaan EA, Pangestuti R, Kim S-K (2018) Seaweeds: Valuable Ingredients for the Pharmaceutical Industries. In: Rampelotto PH, Trincone A (eds) Grand Challenges in Marine Biotechnology. Springer International Publishing, Cham, pp 49–95. https://doi.org/10.1007/978-3-319-69075-9_2
Simic S, Kosanic M, Rankovic B (2012) Evaluation of In Vitro Antioxidant and Antimicrobial Activities of Green Microalgae Trentepohlia umbrina. Not Bot Hort Agrobot Cluj 40:86. https://doi.org/10.15835/nbha4027933
Singh RS, Walia AK (2018) Lectins from red algae and their biomedical potential. J Appl Phycol 30:1833–1858. https://doi.org/10.1007/s10811-017-1338-5
Sommer MOA (2014) Barriers to the spread of resistance. Nature 509:567–568. https://doi.org/10.1038/nature13342
Song C, Yang J, Zhang M, Ding G, Jia C, Qin J, Guo L (2021) Marine Natural Products: The Important Resource of Biological Insecticide. Chem Biodivers 18:e2001020. https://doi.org/10.1002/cbdv.202001020
Sun Y, Chang R, Li Q, Li B (2016) Isolation and characterization of an antibacterial peptide from protein hydrolysates of Spirulina platensis. Eur Food Res Technol 242:685–692. https://doi.org/10.1007/s00217-015-2576-x
Teasdale ME, Shearer TL, Engel S, Alexander TS, Fairchild CR, Prudhomme J, Torres M, Le Roch K, Aalbersberg W, Hay ME, Kubanek J (2012) Bromophycoic Acids: Bioactive Natural Products from a Fijian Red Alga Callophycus sp. J Org Chem 77:8000–8006. https://doi.org/10.1021/jo301246x
Tillotson G (2015) Antimicrobial resistance: what’s needed. Lancet Infect Dis 15:758–760. https://doi.org/10.1016/S1473-3099(15)00081-X
Tolpeznikaite E, Bartkevics V, Ruzauskas M, Pilkaityte R, Viskelis P, Urbonaviciene D, Zavistanaviciute P, Zokaityte E, Ruibys R, Bartkiene E (2021) Characterization of Macro- and Microalgae Extracts Bioactive Compounds and Micro- and Macroelements Transition from Algae to Extract. Foods 10:2226. https://doi.org/10.3390/foods10092226
Tomas M, Capanoglu E, Bahrami A, Hosseini H, Akbari-Alavijeh S, Shaddel R, Rehman A, Rezaei A, Rashidinejad A, Garavand F, Goudarzi M, Jafari SM (2022) The direct and indirect effects of bioactive compounds against coronavirus. Food Front 3:96–123. https://doi.org/10.1002/fft2.119
Usman A, Khalid S, Usman A, Hussain Z, Wang Y (2017) Algal Polysaccharides, Novel Application, and Outlook. In: Zia KM, Zuber M (eds) Algae Based Polymers, Blends, and Composites. Elsevier, pp 115–153. https://doi.org/10.1016/B978-0-12-812360-7.00005-7
Vairappan CS, Suzuki M, Ishii T, Okino T, Abe T, Masuda M (2008) Antibacterial activity of halogenated sesquiterpenes from Malaysian Laurencia spp. Phytochemistry 69:2490–2494. https://doi.org/10.1016/j.phytochem.2008.06.015
Vijayabaskar P, Vaseela N, Thirumaran G (2012) Potential antibacterial and antioxidant properties of a sulfated polysaccharide from the brown marine algae Sargassum swartzii. Chin J Nat Med 10:421–428. https://doi.org/10.1016/S1875-5364(12)60082-X
Wang H, Li Y-L, Shen W-Z, Rui W, Ma X-J, Cen Y-Z (2007) Antiviral activity of a sulfoquinovosyldiacylglycerol (SQDG) compound isolated from the green alga Caulerpa racemosa. botm 50:185–190. https://doi.org/10.1515/BOT.2007.022
Wassie T, Niu K, Xie C, Wang H, Xin W (2021) Extraction Techniques, Biological Activities and Health Benefits of Marine Algae Enteromorpha prolifera Polysaccharide. Front Nutr 8:747928. https://doi.org/10.3389/fnut.2021.747928
Wei Y, Liu Q, Xu C, Yu J, Zhao L, Guo Q (2016) Damage to the Membrane Permeability and Cell Death of Vibrio parahaemolyticus Caused by Phlorotannins with Low Molecular Weight from Sargassum thunbergii. J Aquat Food Prod Technol 25:323–333. https://doi.org/10.1080/10498850.2013.851757
Wells TNC, van Huijsduijnen RH, Van Voorhis WC (2015) Malaria medicines: a glass half full? Nat Rev Drug Discov 14:424–442. https://doi.org/10.1038/nrd4573
Worthington RJ, Melander C (2013) Combination approaches to combat multidrug-resistant bacteria. Trends in Biotechnology 31:177–184. https://doi.org/10.1016/j.tibtech.2012.12.006
Xu X, Piggott AM, Yin L, Capon RJ, Song F (2012) Symphyocladins A–G: bromophenol adducts from a Chinese marine red alga, Symphyocladia latiuscula. Tetrahedron Lett 53:2103–2106. https://doi.org/10.1016/j.tetlet.2012.02.044
Xu X, Yin L, Gao J, Gao L, Song F (2014) Antifungal bromophenols from marine red alga Symphyocladia latiuscula. Chem Biodivers 11:807–811. https://doi.org/10.1002/cbdv.201300239
Xu X, Yin L, Wang Y, Wang S, Song F (2013) A new bromobenzyl methyl sulphoxide from marine red alga Symphyocladia latiuscula. Nat Prod Res 27:723–726. https://doi.org/10.1080/14786419.2012.695362
Yasuhara-Bell J, Lu Y (2010) Marine compounds and their antiviral activities. Antiviral Res 86:231–240. https://doi.org/10.1016/j.antiviral.2010.03.009
Yin S, Davis RA, Shelper T, Sykes ML, Avery VM, Elofsson M, Sundin C, Quinn RJ (2011) Pseudoceramines A–D, new antibacterial bromotyrosine alkaloids from the marine sponge Pseudoceratina sp. Org Biomol Chem 9:6755–6760. https://doi.org/10.1039/C1OB05581J
Yu S-H, Wu S-J, Wu J-Y, Wen D-Y, Mi F-L (2015) Preparation of fucoidan-shelled and genipin-crosslinked chitosan beads for antibacterial application. Carbohydr Polym 126:97–107. https://doi.org/10.1016/j.carbpol.2015.02.068
Zammuto V, Rizzo MG, Spanò A, Spagnuolo D, Di Martino A, Morabito M, Manghisi A, Genovese G, Guglielmino S, Calabrese G, Capparucci F, Gervasi C, Nicolò MS, Gugliandolo C (2022) Effects of crude polysaccharides from marine macroalgae on the adhesion and biofilm formation of Pseudomonas aeruginosa and Staphylococcus aureus. Algal Res 63:102646. https://doi.org/10.1016/j.algal.2022.102646
Zhang H, Khalil Z, Conte MM, Plisson F, Capon RJ (2012) A search for kinase inhibitors and antibacterial agents: bromopyrrolo-2-aminoimidazoles from a deep-water Great Australian Bight sponge, Axinella sp. Tetrahedron Lett 53:3784–3787. https://doi.org/10.1016/j.tetlet.2012.05.051
Acknowledgements
Author (AP) would like to thank Professor Sameer Srivastava (MNNIT Allahabad, India) and Dr Manish P Singh (CDRI Lucknow, India) and Vice Chancellor-AKS University Satna, India for their continuous motivation and support.
Author information
Authors and Affiliations
Contributions
SA: First draft preparation and revise the manuscript, AKY: First draft preparation, AK: Data collection help in first draft preparation, ANY: Review the manuscript at various stage, KC: Reviewing the manuscript at different stage, AP: Conceptualization, Manuscript handling, Revising and review the manuscript at various stage.
Corresponding author
Ethics declarations
Declarations conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Afzal, S., Yadav, A.K., Poonia, A.K. et al. Antimicrobial therapeutics isolated from algal source: retrospect and prospect. Biologia 78, 291–305 (2023). https://doi.org/10.1007/s11756-022-01207-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11756-022-01207-3