Abstract
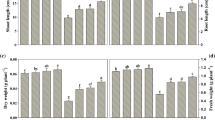
In the current work, the influence of indole-3-acetic acid (IAA; 10–5 M) and 28-homobrassinolide (HBL; 10–8 M) alone or in combination was explored in Solanum melongena L. seedlings treated with sodium chloride (NaCl). The NaCl at different concentrations (5 mM and 15 mM) reduced growth attributes, photosynthetic pigments as well as photosynthesis, and enhanced the dark respiratory rate, and these effects were accompanied with Na+ and reactive oxygen species (ROS) accumulation in leaf tissues. Moreover, NaCl reduced the proficiency of PS II with the increasing doses of selected stress which is apparent from the declined values of Fv/Fm (Phi_P0), Ѱ0 (Psi_0), ϕE0 (Phi_E0) and PIABS, and augmented values of energy fluxes: ABS/RC, ET0/RC, TR0/RC and DI0/RC. The NaCl treatment caused substantial reduction in nitrate and nitrite contents, activities of nitrate reductase, nitrite reductase, glutamine synthetase and glutamate synthase, and increased ammonium content and glutamate dehydrogenase activity. Enhanced level of ROS, i.e. superoxide radical (O2•−) and hydrogen peroxide (H2O2) under NaCl stress amplified the lipid peroxidation product malondialdehyde (MDA), in spite of considerable upsurge in performance of superoxide dismutase, peroxidase, catalase, glutathione-S-transferase enzymes and amount of non-protein thiol, cysteine and proline. Exogenous supplementation of IAA and HBL considerably assuaged the NaCl-induced collapse in growth attributes by manipulating the structural and functional features of photosynthetic apparatus. The ameliorating effect of IAA and HBL, in combination, was due to (1) substantial decrease in Na+ accumulation, (2) improvement in PS II efficiency and nitrogen metabolism and (3) reduced oxidative damage in S. melongena L. due to stimulatory response of antioxidants that keeps the level of oxidants under limit in the seedlings. Thus, the overall results campaigned that IAA and HBL act synergistically to overcome the toxic effects that NaCl had on S. melongena L. seedlings.








Similar content being viewed by others
References
Abouelsaad I, Renault S (2018) Enhanced oxidative stress in the jasmonic acid-deficient tomato mutant def-1 exposed to NaCl stress. J Plant Physiol 226:136–144
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126
Ali Q, Athar HR, Ashraf M (2008) Modulation of growth, photosynthetic capacity and water relations in salt stressed wheat plants by exogenously applied 24-epibrassinolide. Plant Growth Regul 56:107–116
Allen SE, Grimshaw HM, Rowland AP (1986) Chemical analysis. In: Moore PD, Chapman SB (eds) Methods in plant ecology. Blackwell, Oxford, pp 285–344
Bajguz A, Hayat S (2009) Effects of brassinosteroids on the plant responses to environmental stresses. Plant Physiol Biochem 47:1–8
Bates LS, Waldren BP, Teare ID (1973) Rapid determination of free proline of water-stress studies. Plant Soil 39:205–207
Belmecheri-Cherifi H, Albacete A, Martínez-Andújar C, Pérez-Alfocea F, Abrous-Belbachir O (2019) The growth impairment of salinized fenugreek (Trigonella foenum-graecum L.) plants is associated to changes in the hormonal balance. J Plant Physiol 232:311–319
Carillo P, Mastrolonardo G, Nacca F, Fuggi A (2005) Nitrate reductase in durum wheat seedlings as affected by nitrate nutrition and salinity. Funct Plant Biol 32:209–219
Cataldo DA, Haroon M, Schrader LE, Young VL (1975) Rapid colorimetric determination of nitrate in plant tissue by nitration of salicylic acid. Commun Soil Sci Plant Anal 6:71–80
Chrysargyris A, Solomou M, Petropoulos SA, Tzortzakis N (2019) Physiological and biochemical attributes of Mentha spicata when subjected to saline conditions and cation foliar application. J Plant Physiol 232:27–38
Debouba M, Gouia H, Suzuki A, Ghorbel MH (2006) NaCl stress effects on enzymes involved in nitrogen assimilation pathway in tomato Lycopersicon esculentum seedlings. J Plant Physiol 163:1247–1258
Ellman GL (1959) Tissue sulfhydryl groups. Arch Biochem Biophys 82:70–77
Elstner EF, Heupel A (1976) Inhibition of nitrite formation from hydroxylammonium chloride: a simple assay for superoxide dismutase. Anal Biochem 70:616–620
FAO (2012) FAO land and plant nutrition management service. http://faostat.fao.org/site/567/DesktopDefault.aspx?PageID=567#ancor. Accessed 12 May 2012
Frahry G, Schopfer P (2001) NADH-stimulated, cyanide resistant production in maize coleoptiles analyzed with tetrazolium-based assay. Planta 212:175–183
Gaitonde MK (1967) A spectrophotometric method for the direct determination of cysteine in the presence of other naturally occurring amino acids. Biochem J 104:627–633
Giannopolitis CN, Ries SK (1977) Superoxide dismutase. I. Occurrence in higher plants. Plant Physiol 59:309–314
Gill SS, Tuteja N (2010) Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem 48:909–930
Gong B, Wen D, Langenberg KV (2013) Comparative effects of NaCl and NaHCO3 stress on photosynthetic parameters, nutrient metabolism, and the antioxidant system in tomato leaves. Sci Hortic 157:1–12
Habig WH, Pabst MJ, Jakoby WB (1974) Glutathione-S-transferases, the first enzymatic step in mercapturic acid formation. J Biol Chem 249:7130–7139
Hayat S, Hasan SA, Yusuf M, Hayata Q, Aqil Ahmad A (2010) Effect of 28-homobrassinolide on photosynthesis, fluorescence and antioxidant system in the presence or absence of salinity and temperature in Vigna radiata. Environ Exp Bot 69:105–112
Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplasts I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys 125:189–198
Hoagland DR, Arnon DI (1950) The water-culture for growing plants without soil. Calif Agric Exp Station Circ 347:1–39
Hussain A, Nazir F, Fariduddin Q (2019) 24-Epibrassinolide and spermidine alleviate Mn stress via the modulation of root morphology, stomatal behavior, photosynthetic attributes and antioxidant defense in Brassica juncea. Physiol Mol Biol Plants. https://doi.org/10.1007/s12298-019-00672-6
Kumar J, Singh S, Singh M, Srivastava PK, Mishra RK, Singh VP, Prasad SM (2017) Transcriptional regulation of salinity stress in plants: a short review. Plant Gene 11:160–169
Kurra-Hotta M, Satoh K, Katoh S (1987) Relationship between photosynthesis and chlorophyll content during leaf senescence of rice seedlings. Plant Cell Physiol 28:1321–1329
Lichtenthaler HK (1987) Chlorophylls and carotenoids: pigments of photosynthetic biomembranes. Methods Enzymol 148:350–382
Lillo C (1984) Diurnal variations of nitrite reductase, glutamine synthetase, glutamate synthase, alanine amino transferase and aspartate amino transferase in barley leaves. Physiol Plant 6:214–218
Molins-Legua C, Meseguer-Lloret S, Moliner-Martinez Y, Campıns-Falco P (2006) Aguide for selecting the most appropriate method for ammonium determination in water analysis. Trends Anal Chem 20:282–290
Nemhauser JL, Mockler TC, Chory J (2004) Interdependency of brassinosteroid and auxin signaling in Arabidopsis. PLoS Biol 2:E258
Parihar P, Singh S, Singh R, Singh VP, Prasad SM (2015) Effect of salinity stress on plants and its tolerance strategies: a review. Environ Sci Pollut Res 22:4056–4075
Pompella A, Maellaro E, Casini AF, Comporti M (1987) Histochemical detection of lipid peroxidation in the liver of bromobenzene-poisoned mice. Am J Pathol 129:295–301
Poór P, Borbély P, Czékus Z, Takács Z, Ördög A, Popović B, Taria I (2019) Comparison of changes in water status and photosynthetic parameters in wild type and abscisic acid-deficient sitiens mutant of tomato (Solanum lycopersicum cv. Rheinlands Ruhm) exposed to sublethal and lethal salt stress. J Plant Physiol 232:130–140
Qadir M, Oster JD, Schubert S, Noble AD, Sahrawat KL (2007) Phytoremediation of sodic and saline–sodic soils. Adv Agron 96:197–247
Robin P (1979) Etude de quelques conditions d’extraction de la nitrate rèductase des racines et des feuilles de plantules de maïs. PhysiolVèg 17:45–54
Siddiqui MH, Mohammad F, Masrooor M, Khan A, Al-Whaibi MH (2012) Cumulative effect of nitrogen and sulphur on Brassica juncea L. genotypes under NaCl stress. Protoplasma 249:139–153
Singh M, Kumar J, Singh VP, Prasad SM (2014) Plant tolerance mechanism against salt stress: the nutrient management approach. Biochem Pharmacol 3:165
Singh M, Kumar J, Singh S, Singh VP, Prasad SM (2015) Roles of osmoprotectants in improving salinity and drought tolerance in plants: a review. Rev Environ Sci Biotechnol. https://doi.org/10.1007/s11157-015-9372-8
Singh M, Singh VP, Prasad SM (2016) Responses of photosynthesis, nitrogen and proline metabolism to salinity stress in Solanum lycopersicum under different levels of nitrogen supplementation. Plant Physiol Biochem 109:72–83
Singh RP, Srivastava HS (1983) Regulation of glutamate dehydrogenase activity by amino acids in maize seedlings. Physiol Plant 57:549–554
Singh RP, Srivastava HS (1986) Increase in glutamate synthase (NADH) activity in maize seedlings in response to nitrate and ammonium nitrogen. Physiol Plant 66:413–416
Singh S, Prasad SM (2015) IAA alleviates Cd toxicity on growth, photosynthesis and oxidative damages in eggplant seedlings. Plant Growth Regul 77:87–98
Singh S, Prasad SM (2017) Effects of 28-homobrassinoloid on key physiological attributes of Solanum lycopersicum seedlings under cadmium stress: photosynthesis and nitrogen metabolism. Plant Growth Regul 82:161–173
Sirhindi G, Kumar S, Bhardwaj R, Kumar M (2009) Effects of 24-epibrassinolide and 28-homobrassinolide on the growth and antioxidant enzyme activities in the seedlings of Brassica juncea L. Physiol Mol Biol Plants 15:335–341
Snell FD, Snell CT (1949) Colorimetric methods of analysis, vol 3. Van Nostrand, New York, pp 804–805
Strasser RJ, Srivastava A, Tsimilli-Michael M (2000) The fluorescence transient as a tool to characterise and screen photosynthetic samples. In: Yunus M, Pathre U, Mohanty P (eds) Probing photosynthesis: mechanisms, regulation and adaptation. Taylor and Francis, London, pp 445–483
Taïbi K, Taïbi F, Abderrahim LA, Ennajah A, Belkhodja M, Mulet JM (2016) Effect of salt stress on growth, chlorophyll content, lipid peroxidation and antioxidant defence systems in Phaseolus vulgaris L. S Afr J Bot 105:306–312
Thordal-Christensen H, Zhang Z, Wei Y, Collinge DB (1997) Subcellular localization of H2O2 in plants, H2O2 accumulation in papillae and hypersensitive response during barley powdery mildew interaction. Plant J 11:1187–1194
Tofighi C, Khavari-Nejad RA, Najafi F, Razavi K, Rejali F (2017) Responses of wheat plants to interactions of 24-epibrassinolide and Glomus mosseae in saline condition. Physiol Mol Biol Plants 23:557–564
Velikova V, Yordanov I, Edreva A (2000) Oxidative stress and some antioxidant system in acid rain-treated bean plants. Plant Sci 151:59–66
Vert G, Walcher CL, Chory J, Nemhauser JL (2008) Integration of auxin and brassinosteroid pathways by auxin response factor 2. Proc Natl Acad Sci USA 105:9829–9834
Wang H, Shan X, Wen B, Owens G, Fang J, Zhang S (2007) Effect of indole acetic acid on lead accumulation in maize (Zea mays L.) seedlings and relevant antioxidant response. Environ Exp Bot 61:246–253
Xia JX, Huang LF, Zhou YH, Mao HW, Shi K, Wu JX, Asami T, Chen ZX, Yu JQ (2009) Brassinosteroids promote photosynthesis and growth by enhancing activation of Rubisco and expression of photosynthetic genes in Cucumis sativus. Planta 230:1185–1196
Yusuf M, Fariduddin Q, Ahmad I, Ahmad A (2014) Brassinosteroid-mediated evaluation of antioxidant system and nitrogen metabolism in two contrasting cultivars of Vigna radiata under different levels of nickel. Physiol Mol Biol Plants 20:449–460
Zeng H, Tang Q, Hua X (2010) Arabidopsis brassinosteroid mutants det 2–1 and bin 2–1 display altered salt tolerance. J Plant Growth Regul 29:44–52
Zhang XZ (1992) The measurement and mechanism of lipid peroxidation and SOD, POD and CAT activities in biological system. In: Zhang XZ (ed) Research method crop physiology. Agriculture Press, Beijing, pp 208–211
Zhu YF, Wu YX, Hu Y, Jia XM, Zhao T, Cheng L, Wang YX (2019) Tolerance of two apple rootstocks to short-term salt stress: focus on chlorophyll degradation, photosynthesis, hormone and leaf ultrastructures. Acta Physiol Plant 41:87. https://doi.org/10.1007/s11738-019-2877-y
Acknowledgements
Aman Deep Raju is grateful to the CSIR-UGC (JRF; letter no. 2061631013) for getting financial assistance as SRF to conduct the present piece of work. Professor Sheo Mohan Prasad is also thankful to the Department of Science and Technology (DST), New Delhi, for getting research amenities under DST‒FIST program.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Communicated by Z.-L. Zhang.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Raju, A.D., Parihar, P., Singh, R. et al. Synergistic action of indole acetic acid with homobrassinolide in easing the NaCl-induced toxicity in Solanum melongena L. seedlings. Acta Physiol Plant 42, 68 (2020). https://doi.org/10.1007/s11738-020-03054-8
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11738-020-03054-8