Abstract
The control of scion vigor by dwarfing apple rootstocks is most convincingly elucidated by the shoot–root–shoot signaling of auxins and other hormones. To identify auxin and auxin-related genes that may play roles in the composite tree’s auxin metabolism and dwarfing mechanism, the concentrations of IAA and the expression level of key auxin synthesis, transport, and metabolism genes were measured in leaves, phloem, and roots from the dwarfing Fuji/M9 and the vigorous Fuji/MM106. The results showed that the indole-3-acetic acid (IAA) content was lower in the dwarfing Fuji/M9 than in the vigorous Fuji/MM106. The IAA content in the Fuji/M9 rootstock’s phloem was higher than that of its scion phloem. The expression level of MdYUCCA10a gene was significantly lower in the leaves and roots of Fuji/M9 than in that of the Fuji/MM106. The phloem and roots of the Fuji/M9 rootstock showed low expression levels of MdPIN1b and MdPIN8a. The auxin-conjugated genes MdGH3-5b and MdGH3-9a showed lower expression levels in the Fuji/M9 than in the Fuji/MM106. However, the Fuji/M9 showed higher levels of the auxin-conjugate hydrolase genes MdIAR3c and MdILL6c. The low expression level of auxin synthesis gene MdYUCCA10a in Fuji/M9 probably induced the low auxin level. The lower expression levels of auxin transport genes MdPIN1b and MdPIN8a in the M9 rootstock were suggested to probably contribute to auxin accumulation in Fuji/M9 rootstock phloem. The low amount of auxin transported from the shoots along with the root auxin synthesis deficiencies reduced the root growth and then decreased the supply of root-produced substances to the shoots in Fuji/M9.








Similar content being viewed by others
References
Alvarez R, Nissen SJ, Sutter EG (1989) Relationship between indole-3-acetic acid levels in apple (Malus pumila Mill) rootstocks cultured in vitro and adventitious root formation in the presence of indole-3-butyric acid. Plant Physiol 89:439–443
Atkinson C, Else M, Taylor L, Dover C (2003) Root and stem hydraulic conductivity as determinants of growth potential in grafted trees of apple (Malus pumila Mill.). J Exp Bot 54:1221–1229
Blakeslee JJ, Peer WA, Murphy AS (2005) Auxin transport. Curr Opin Plant Biol 8:494–500
Chandler JW (2009) Auxin as compère in plant hormone crosstalk. Planta 231:1–12
Chen Q, Dai X, De-Paoli H, Cheng Y, Takebayashi Y, Kasahara H, Kamiya Y, Zhao Y (2014) Auxin overproduction in shoots cannot rescue auxin deficiencies in Arabidopsis roots. Plant Cell Physiol 55:1072–1079
Cornille A, Giraud T, Smulders MJ, Roldán-Ruiz I, Gladieux P (2014) The domestication and evolutionary ecology of apples. Trends Genet 30:57–65
Dal Bosco C, Dovzhenko A, Liu X, Woerner N, Rensch T, Eismann M, Eimer S, Hegermann J, Paponov IA, Ruperti B, Heberle-Bors E, Touraev A, Cohen JD, Palme K (2012) The endoplasmic reticulum localized PIN8 is a pollen-specific auxin carrier involved in intracellular auxin homeostasis. Plant J 71:860–870
Dal Cin V, Velasco R, Ramina A (2009) Dominance induction of fruitlet shedding in Malus × domestica (L Borkh): molecular changes associated with polar auxin transport. BMC Plant Biol 9:139
Ding X, Cao Y, Huang L, Zhao J, Xu C, Li X, Wang S (2008) Activation of the indole-3-acetic acid-amido synthetase GH3-8 suppresses expansin expression and promotes salicylate- and jasmonate-independent basal immunity in rice. Plant Cell 20:228–240
Ding Z, Wang B, Moreno I, Duplakova N, Simon S, Carraro N, Reemmer J, Pencik A, Chen X, Tejos R, Skupa P, Pollmann S, Mravec J, Petrasek J, Zazimalova E, Honys D, Rolcik J, Murphy A, Orellana A, Geisler M, Friml J (2012) ER-localized auxin transporter PIN8 regulates auxin homeostasis and male gametophyte development in Arabidopsis. Nat Commun 3:941
Dobrev PI, Kamínek M (2002) Fast and efficient separation of cytokinins from auxin and abscisic acid and their purification using mixed-mode solid-phase extraction. J Chromatogr A 950:21–29
Dobrev PI, Havlíček L, Vágner M, Malbeck J, Kamínek M (2005) Purification and determination of plant hormones auxin and abscisic acid using solid phase extraction and two-dimensional high performance liquid chromatography. J Chromatogr A 1075:159–166
Mount DW (2007) Using the Basic Local Alignment Search Tool (BLAST). CSH Protoc 2007
Finn RD, Bateman A, Clements J, Coggill P, Eberhardt RY, Eddy SR, Heger A, Hetherington K, Holm L, Mistry J, Sonnhammer EL, Tate J, Punta M (2014) Pfam: the protein families database. Nucleic Acids Res 42:D222–D2230
Gur A, Samish R (1968) The role of auxins and auxin destruction in the vigor effect induced by various apple rootstocks. Beitrage Biol Pfl 45:91–111 (bibl. 47)
Hedden P (2003) The genes of the Green Revolution. Trends Genet 19:5–9
Jung S, Ficklin SP, Lee T, Cheng CH, Blenda A, Zheng P, Yu J, Bombarely A, Cho I, Ru S, Evans K, Peace C, Abbott AG, Mueller LA, Olmstead MA, Main D (2014) The Genome Database for Rosaceae (GDR): year 10 update. Nucleic Acids Res 42:D1237–D1244
Kamboj JS, Quinlan JD (1998) The apple rootstock and its influence on endogenous hormones. Acta Hortic 463:143–152
Krecek P, Skupa P, Libus J, Naramoto S, Tejos R, Friml J, Zazimalova E (2009) The PIN-FORMED (PIN) protein family of auxin transporters. Genome Biol 10
Lauri PE, Maguylo K, Trottier C (2006) Architecture and size relations: an essay on the apple (Malus × domestica, Rosaceae) tree. Am J Bot 93:357–368
Leclere S, Tellez R, Rampey RA, Matsuda SPT, Bartel B (2002) Characterization of a family of IAA-amino acid conjugate hydrolases from Arabidopsis. J Biol Chem 277:20446–20452
Letunic I, Doerks T, Bork P (2012) SMART 7: recent updates to the protein domain annotation resource. Nucleic Acids Res 40:D302–D305
Li LC, Qin GJ, Tsuge T, Hou XH, Ding MY, Aoyama T, Oka A, Chen Z, Gu H, Zhao Y, Qu LJ (2008) SPOROCYTELESS modulates YUCCA expression to regulate the development of lateral organs in Arabidopsis. New Phytol 179:751–764
Lochard R, Schneider G (1981) Stock and scion growth relationships and the dwarfing mechanism in apple. Hortic Rev 3:315–375
Mano Y, Nemoto K (2012) The pathway of auxin biosynthesis in plants. J Exp Bot 63:2853–2872
Mashiguchi K, Tanaka K, Sakai T, Sugawara S, Kawaide H, Natsume M, Hanada A, Yaeno T, Shirasu K, Yao H, McSteen P, Zhao YD, Hayashi K, Kamiya Y, Kasahara H (2011) The main auxin biosynthesis pathway in Arabidopsis. Proc Natl Acad Sci USA 108:18512–18517
Michalczuk L (2002) Indole-3-acetic acid level in wood, bark and cambial sap of apple rootstocks differing in growth vigour. Acta Physiol Plant 24:131–136
Mravec J, Skupa P, Bailly A, Hoyerova K, Krecek P, Bielach A, Petrasek J, Zhang J, Gaykova V, Stierhof YD, Dobrev PI, Schwarzerova K, Rolcik J, Seifertova D, Luschnig C, Benkova E, Zazimalova E, Geisler M, Friml J (2009) Subcellular homeostasis of phytohormone auxin is mediated by the ER-localized PIN5 transporter. Nature 459:1136–1140
Ostrowski M, Jakubowska A (2008) Identification of enzyme activity that conjugates indole-3-acetic acid to aspartate in immature seeds of pea (Pisum sativum). J Plant Physiol 165(5):564–569
Ostrowski M, Jakubowska A (2011) Purification and biochemical characterization of indole-3-acetyl-aspartic acid synthetase from immature seeds of pea (Pisum sativum). J Plant Growth Regul 30(1):30–40
Rampey RA, LeClere S, Kowalczyk M, Ljung K, Sandberg G, Bartel B (2004) A family of auxin-conjugate hydrolases that contributes to free indole-3-acetic acid levels during Arabidopsis germination. Plant Physiol 135:978–988
Robinson T, Aldwinckle H, Fazio G, Holleran T (2002) The Geneva series of apple rootstocks from Cornell: performance, disease resistance, and commercialization, In: XXVI international horticultural congress: genetics and breeding of tree fruits and nuts, vol 622, pp 513–520
Saeed AI, Sharov V, White J, Li J, Liang W, Bhagabati N, Braisted J, Klapa M, Currier T, Thiagarajan M, Sturn A, Snuffin M, Rezantsev A, Popov D, Ryltsov A, Kostukovich E, Borisovsky I, Liu Z, Vinsavich A, Trush V, Quackenbush J (2003) TM4: a free, open-source system for microarray data management and analysis. Biotechniques 34:374–378
Seleznyova AN, Thorp TG, White M, Tustin S, Costes E (2003) Application of architectural analysis and AMAPmod methodology to study dwarfing phenomenon: the branch structure of ‘Royal Gala’ apple grafted on dwarfing and non-dwarfing rootstock/interstock combinations. Ann Bot 91:665–672
Seleznyova AN, Tustin DS, Thorp TG (2008) Apple dwarfing rootstocks and interstocks affect the type of growth units produced during the annual growth cycle: precocious transition to flowering affects the composition and vigour of annual shoots. Ann Bot 101:679–687
Shehata SAM, El-Shraiy AM (2010) Regulating cucumber grafting by interactions of cytokinins in xylem exudates of rootstock and basipetal polar auxin transport of scion at graft union. Aust J Basic Appl Sci 4:6179–6184
Singh Kamboj J, Quinlan JD (1997) The apple rootstock and its influence on endogenous hormones. In: VIII international symposium on plant bioregulation in fruit production, vol 463, pp 143–152
Soejima J, Yoshida Y, Haniuda T, Bessho H, Tsuchiya S, Masuda T, Komori S, Sanada T, Ito Y, Sadamori S (2010) New dwarfing apple rootstocks ‘JM1’, ‘JM7’ and ‘JM8’. Bull Natl Inst Fruit Tree Sci 11:1–16
Soumelidou K, Battey N, John P, Barnett J (1994a) The anatomy of the developing bud union and its relationship to dwarfing in apple. Ann Bot 74:605–611
Soumelidou K, Morris D, Battey N, Barnett J, John P (1994b) Auxin transport capacity in relation to the dwarfing effect of apple rootstocks. J Hortic Sci 69:719–725
Spiess GM, Hausman A, Yu P, Cohen JD, Rampey RA, Zolman BK (2014) Auxin input pathway disruptions are mitigated by changes in auxin biosynthetic gene expression in Arabidopsis. Plant Physiol 165:1092–1104
Staswick PE, Serban B, Rowe M, Tiryaki I, Maldonado MT, Maldonado MC, Suza W (2005) Characterization of an Arabidopsis enzyme family that conjugates amino acids to indole-3-acetic acid. Plant Cell 17(2):616–627
Van Hooijdonk B, Woolley D, Warrington I, Tustin D (2010) Initial alteration of scion architecture by dwarfing apple rootstocks may involve shoot–root–shoot signalling by auxin, gibberellin, and cytokinin. J Hortic Sci Biotechnol 85:59–65
Van Hooijdonk B, Woolley D, Warrington I, Tustin D (2011) Rootstocks modify scion architecture, endogenous hormones, and root growth of newly grafted ‘Royal Gala’ apple trees. J Am Soc Hortic Sci 136:93–102
Won C, Shen X, Mashiguchi K, Zheng Z, Dai X, Cheng Y, Kasahara H, Kamiya Y, Chory J, Zhao Y (2011) Conversion of tryptophan to indole-3-acetic acid by TRYPTOPHAN AMINOTRANSFERASES OF ARABIDOPSIS and YUCCAs in Arabidopsis. Proc Natl Acad Sci USA 108:18518–18523
Yildirim AN, Polat M, Dolgun O, Askin MA, Gokbayrak Z, San B (2010) Graft formation in some spur and vigorous apple varieties grafted on Ottawa 3 rootstock: a histological investigation. J Food Agric Environ 8:512–514
Yin H, Yan B, Sun J, Jia P, Zhang Z, Yan X, Chai J, Ren Z, Zheng G, Liu H (2012) Graft-union development: a delicate process that involves cell–cell communication between scion and stock for local auxin accumulation. J Exp Bot 63:4219–4232
Zagaja S (1980) Performance of two apple cultivars on P series dwarf rootstocks. In: Symposium on research and development on orchard and plantation systems, vol 114, pp 162–169
Zhang SW, Li CH, Cao J, Zhang YC, Zhang SQ, Xia YF, Sun DY, Sun Y (2009) Altered architecture and enhanced drought tolerance in rice via the down-regulation of indole-3-acetic acid by TLD1/OsGH3.13 activation. Plant Physiol 151:1889–1901
Zhao YD (2008) The role of local biosynthesis of auxin and cytokinin in plant development. Curr Opin Plant Biol 11:16–22
Zhao Y (2010) Auxin biosynthesis and its role in plant development. Annu Rev Plant Biol 61:49–64
Zhao Y, Christensen SK, Fankhauser C, Cashman JR, Cohen JD, Weigel D, Chory J (2001) A role for flavin monooxygenase-like enzymes in auxin biosynthesis. Science 291:306–309
Acknowledgments
This study was sponsored by the National Science and Technology Supporting Project (2013BAD20B03), National Apple Industry Technology System of Agriculture Ministry of China (CARS-28), National Spark Plan Program (2014GA850002), Science and Technology Innovative Engineering Project in Shaanxi province of China (2015NY114), Yangling Subsidiary Center Project of National Apple Improvement Center, and Collaborative Innovation of Center Shaanxi Fruit Industry Development, Innovation project of science and technology plan projects of Shaanxi province (2016TZC-N-11-6).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by W. Wang.
C. Song and D. Zhang contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
11738_2016_2065_MOESM1_ESM.tif
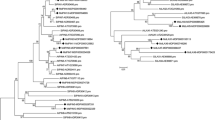
Figure S1 Phylogenetic tree of Malus domestica and Arabidopsis YUCCA proteins. The amino acid sequences of the YUCCA proteins were aligned with ClustalW, and the phylogenetic tree was constructed using the neighbor-joining method of MEGA 5.0 software. Each node is represented by a number that indicates the bootstrap value for 1000 replicates. The scale bar represents 0.05 substitutions per sequence position. (TIFF 3025 kb)
11738_2016_2065_MOESM2_ESM.tif
Figure S2 Phylogenetic tree of Malus domestica and Arabidopsis PIN proteins. The amino acid sequences of the PIN proteins were aligned with ClustalW, and the phylogenetic tree was constructed using the neighbor-joining method of MEGA 5.0 software. Each node is represented by a number that indicates the bootstrap value for 1000 replicates. The scale bar represents 0.1 substitutions per sequence position (TIFF 3792 kb)
11738_2016_2065_MOESM3_ESM.tif
Figure S3 Phylogenetic tree of Malus domestica and Arabidopsis GH3 proteins. The amino acid sequences of the GH3 proteins were aligned with ClustalW, and the phylogenetic tree was constructed using the neighbor-joining method of MEGA 5.0 software. Each node is represented by a number that indicates the bootstrap value for 1000 replicates. The scale bar represents 0.05 substitutions per sequence position (TIFF 204 kb)
11738_2016_2065_MOESM4_ESM.jpg
Figure S4 Phylogenetic tree of Malus domestica and Arabidopsis ILR proteins. The amino acid sequences of the ILR proteins were aligned with ClustalW, and the phylogenetic tree was constructed using the neighbor-joining method of MEGA 5.0 software. Each node is represented by a number that indicates the bootstrap value for 1000 replicates. The scale bar represents 0.1 substitutions per sequence position (JPEG 1353 kb)
11738_2016_2065_MOESM6_ESM.emf
Figure S6 Multiple sequence alignment of the MdPIN proteins obtained using DNAMAN software. Mem_trans is the consensus sequence of the Mem_trans domain family (PF03547.13) (EMF 5304 kb)
11738_2016_2065_MOESM7_ESM.emf
Figure S7 Multiple sequence alignment of the MdGH3 proteins obtained using DNAMAN software. GH3 is the consensus sequence of the GH3 domain family (PF03321.8) (EMF 1855 kb)
11738_2016_2065_MOESM8_ESM.emf
Figure S8 Multiple sequence alignment of the MdILR proteins obtained using DNAMAN software. Peptidase_M20 is the consensus sequence of the Peptidase_M20 domain family (PF01546.23) (EMF 4638 kb)
11738_2016_2065_MOESM12_ESM.tif
Figure S12 Expression of auxin-conjugate hydrolase genes in the shoot tip (A), phloem (B) and root (C) of M9 and MM106 (TIFF 3392 kb)
Rights and permissions
About this article
Cite this article
Song, C., Zhang, D., Zhang, J. et al. Expression analysis of key auxin synthesis, transport, and metabolism genes in different young dwarfing apple trees. Acta Physiol Plant 38, 43 (2016). https://doi.org/10.1007/s11738-016-2065-2
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11738-016-2065-2