Abstract
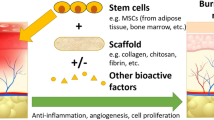
Tissue engineering focuses on repairing tissue and restoring tissue functions by employing three elements: scaffolds, cells and biochemical signals. In tissue engineering, bioactive material scaffolds have been used to cure tissue and organ defects with stem cell-based therapies being one of the best documented approaches. In the review, different biomaterials which are used in several methods to fabricate tissue engineering scaffolds were explained and show good properties (biocompatibility, biodegradability, and mechanical properties etc.) for cell migration and infiltration. Stem cell homing is a recruitment process for inducing the migration of the systemically transplanted cells, or host cells, to defect sites. The mechanisms and modes of stem cell homing-based tissue engineering can be divided into two types depending on the source of the stem cells: endogenous and exogenous. Exogenous stem cell-based bioactive scaffolds have the challenge of long-term culturing in vitro and for endogenous stem cells the biochemical signal homing recruitment mechanism is not clear yet. Although the stem cell homing-based bioactive scaffolds are attractive candidates for tissue defect therapies, based on in vitro studies and animal tests, there is still a long way before clinical application.
Similar content being viewed by others
References
Nucera S, Biziato D, De Palma M. The interplay between macrophages and angiogenesis in development, tissue injury and regeneration. The International Journal of Developmental Biology, 2011, 55(4–5): 495–503
Tanaka H, Sugimoto H, Yoshioka T, et al. Role of granulocyte elastase in tissue injury in patients with septic shock complicated by multiple-organ failure. Annals of Surgery, 1991, 213(1): 81–85
Chancellor M B, Huard J, Capelli C, et al. Rapid preparation of stem cell matrices for use in tissue and organ treatment and repair. European Patent, EP1372398, 2013-07-10
Schrier R W, Parikh C R. Comparison of renal injury in myeloablative autologous, myeloablative allogeneic and nonmyeloablative allogeneic haematopoietic cell transplantation. Nephrology, Dialysis, Transplantation, 2005, 20(4): 678–683
Battiston B, Geuna S, Ferrero M, et al. Nerve repair by means of tubulization: literature review and personal clinical experience comparing biological and synthetic conduits for sensory nerve repair. Microsurgery, 2005, 25(4): 258–267
Wiria F E, Leong K F, Chua C K, et al. Poly-e-caprolactone/ hydroxyapatite for tissue engineering scaffold fabrication via selective laser sintering. Acta Biomaterialia, 2007, 3(1): 1–12
Luo Y, Shoichet M S. A photolabile hydrogel for guided three-dimensional cell growth and migration. Nature Materials, 2004, 3(4): 249–253
Atala A. Engineering tissues, organs and cells. Journal of Tissue Engineering and Regenerative Medicine, 2007, 1(2): 83–96
Hutmacher D W, Sittinger M, Risbud M V. Scaffold-based tissue engineering: rationale for computer-aided design and solid freeform fabrication systems. Trends in Biotechnology, 2004, 22(7): 354–362
Meinel L, Karageorgiou V, Fajardo R, et al. Bone tissue engineering using human mesenchymal stem cells: effects of scaffold material and medium flow. Annals of Biomedical Engineering, 2004, 32(1): 112–122
Giannobile WV. Periodontal tissue engineering by growth factors. Bone, 1996, 19(1 Suppl): 23–37
Ito Y. Tissue engineering by immobilized growth factors. Materials Science and Engineering C, 1998, 6(4): 267–274
Gallagher K A, Liu Z J, Xiao M, et al. Diabetic impairments in NO-mediated endothelial progenitor cell mobilization and homing are reversed by hyperoxia and SDF-1a. The Journal of Clinical Investigation, 2007, 117(5): 1249–1259
Wojakowski W, Kucia M, Milewski K, et al. The role of CXCR4/ SDF-1, CD117/SCF, and c-met/HGF chemokine signalling in the mobilization of progenitor cells and the parameters of the left ventricular function, remodelling, and myocardial perfusion following acute myocardial infarction. European Heart Journal Supplements, 2008, 10(suppl K): K16–K23
Schenk S, Mal N, Finan A, et al. Monocyte chemotactic protein-3 is a myocardial mesenchymal stem cell homing factor. Stem Cells, 2007, 25(1): 245–251
Chen F M, Zhang M,Wu Z F. Toward delivery of multiple growth factors in tissue engineering. Biomaterials, 2010, 31(24): 6279–6308
Brody S, Pandit A. Approaches to heart valve tissue engineering scaffold design. Journal of Biomedical Materials Research Part B: Applied Biomaterials, 2007, 83B(1): 16–43
Gao C, Wan Y, Yang C, et al. Preparation and characterization of bacterial cellulose sponge with hierarchical pore structure as tissue engineering scaffold. Journal of Porous Materials, 2011, 18(2): 139–145
Jha B S, Ayres C E, Bowman J R, et al. Electrospun collagen: a tissue engineering scaffold with unique functional properties in a wide variety of applications. Journal of Nanomaterials, 2011, (15): 367–371
Zhu H, Ji J, Shen J. Biomacromolecules electrostatic selfassembly on 3-dimensional tissue engineering scaffold. Biomacromolecules, 2004, 5(5): 1933–1939
McManus M C, Boland E D, Simpson D G, et al. Electrospun fibrinogen: feasibility as a tissue engineering scaffold in a rat cell culture model. Journal of Biomedical Materials Research Part A, 2007, 81(2): 299–309
Chen Q Z, Thompson I D, Boccaccini A R. 45S5 Bioglass-derived glass–ceramic scaffolds for bone tissue engineering. Biomaterials, 2006, 27(11): 2414–2425
Xu T, Miszuk J M, Zhao Y, et al. Electrospun polycaprolactone 3D nanofibrous scaffold with interconnected and hierarchically structured pores for bone tissue engineering. Advanced Healthcare Materials, 2015, 4(15): 2238–2246
Yin G B, Zhang Y Z, Wang S D, et al. Study of the electrospun PLA/silk fibroin-gelatin composite nanofibrous scaffold for tissue engineering. Journal of Biomedical Materials Research Part A, 2010, 93(1): 158–163
Sakimura K, Matsumoto T, Miyamoto C, et al. Effects of insulinlike growth factor I on transforming growth factor ß1 induced chondrogenesis of synovium-derived mesenchymal stem cells cultured in a polyglycolic acid scaffold. Cells, Tissues, Organs, 2006, 183(2): 55–61
Ma Z, Gao C, Gong Y, et al. Cartilage tissue engineering PLLA scaffold with surface immobilized collagen and basic fibroblast growth factor. Biomaterials, 2005, 26(11): 1253–1259
Park S A, Lee S H, Kim W D. Fabrication of porous polycaprolactone/hydroxyapatite (PCL/HA) blend scaffolds using a 3D plotting system for bone tissue engineering. Bioprocess and Biosystems Engineering, 2011, 34(4): 505–513
Kim S H, Kwon J H, Chung M S, et al. Fabrication of a new tubular fibrous PLCL scaffold for vascular tissue engineering. Journal of Biomaterials Science. Polymer Edition, 2006, 17(12): 1359–1374
Rockwood D N, Preda R C, Yücel T, et al. Materials fabrication from Bombyx mori silk fibroin. Nature Protocols, 2011, 6(10): 1612–1631
Yang J W, Zhang Y F, Sun Z Y, et al. Dental pulp tissue engineering with bFGF-incorporated silk fibroin scaffolds. Journal of Biomaterials Applications, 2015, 30(2): 221–229
Zhang K, Wang H, Huang C, et al. Fabrication of silk fibroin blended P(LLA-CL) nanofibrous scaffolds for tissue engineering. Journal of Biomedical Materials Research Part A, 2010, 93(3): 984–993
Prabhakaran M P, Venugopal J R, Chyan T T, et al. Electrospun biocomposite nanofibrous scaffolds for neural tissue engineering. Tissue Engineering Part A, 2008, 14(11): 1787–1797
Courtney T, Sacks M S, Stankus J, et al. Design and analysis of tissue engineering scaffolds that mimic soft tissue mechanical anisotropy. Biomaterials, 2006, 27(19): 3631–3638
Burdick J A, Anseth K S. Photoencapsulation of osteoblasts in injectable RGD-modified PEG hydrogels for bone tissue engineering. Biomaterials, 2002, 23(22): 4315–4323
Sill T J, von Recum H A. Electrospinning: applications in drug delivery and tissue engineering. Biomaterials, 2008, 29(13): 1989–2006
Huang Z M, Zhang Y Z, Kotaki M, et al. A review on polymer nanofibers by electrospinning and their applications in nanocomposites. Composites Science and Technology, 2003, 63(15): 2223–2253
Jin H J, Chen J, Karageorgiou V, et al. Human bone marrow stromal cell responses on electrospun silk fibroin mats. Biomaterials, 2004, 25(6): 1039–1047
Panseri S, Cunha C, Lowery J, et al. Electrospun micro- and nanofiber tubes for functional nervous regeneration in sciatic nerve transections. BMC Biotechnology, 2008, 8(1): 39
Wang C Y, Liu J J, Fan C Y, et al. The effect of aligned core–shell nanofibres delivering NGF on the promotion of sciatic nerve regeneration. Journal of Biomaterials Science: Polymer Edition, 2012, 23(1–4): 167–184
Keshaw H, Thapar N, Burns A J, et al. Microporous collagen spheres produced via thermally induced phase separation for tissue regeneration. Acta Biomaterialia, 2010, 6(3): 1158–1166
Chun KW, Cho K C, Kim S H, et al. Controlled release of plasmid DNA from biodegradable scaffolds fabricated using a thermallyinduced phase-separation method. Journal of Biomaterials Science: Polymer Edition, 2004, 15(11): 1341–1353
Ma H, Hu J, Ma P X. Polymer scaffolds for small-diameter vascular tissue engineering. Advanced Functional Materials, 2010, 20(17): 2833–2841
Kim M, Kim G H. Electrohydrodynamic direct printing of PCL/ collagen fibrous scaffolds with a core/shell structure for tissue engineering applications. Chemical Engineering Journal, 2015, 279: 317–326
Lee J W, Choi Y J, Yong W J, et al. Development of a 3D cell printed construct considering angiogenesis for liver tissue engineering. Biofabrication, 2016, 8(1): 015007
Goole J, Amighi K. 3D printing in pharmaceutics: A new tool for designing customized drug delivery systems. International Journal of Pharmaceutics, 2016, 499(1–2): 376–394
Beltrami A P, Barlucchi L, Torella D, et al. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell, 2003, 114(6): 763–776
Daley G Q, Scadden D T. Prospects for stem cell-based therapy. Cell, 2008, 132(4): 544–548
Sieveking D P, Ng M K C. Cell therapies for therapeutic angiogenesis: back to the bench. Vascular Medicine, 2009, 14(2): 153–166
Bajada S, Mazakova I, Richardson J B, et al. Updates on stem cells and their applications in regenerative medicine. Journal of Tissue Engineering and Regenerative Medicine, 2008, 2(4): 169–183
Teo A K K, Vallier L. Emerging use of stem cells in regenerative medicine. The Biochemical Journal, 2010, 428(1): 11–23
Quesenberry P J, Becker P S. Stem cell homing: rolling, crawling, and nesting. Proceedings of the National Academy of Sciences of the United States of America, 1998, 95(26): 15155–15157
Khaldoyanidi S. Directing stem cell homing. Cell Stem Cell, 2008, 2(3): 198–200
Nakatomi H, Kuriu T, Okabe S, et al. Regeneration of hippocampal pyramidal neurons after ischemic brain injury by recruitment of endogenous neural progenitors. Cell, 2002, 110(4): 429–441
Méndez-Ferrer S, Michurina T V, Ferraro F, et al. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature, 2010, 466(7308): 829–834
Chen F M, Zhang J, Zhang M, et al. A review on endogenous regenerative technology in periodontal regenerative medicine. Biomaterials, 2010, 31(31): 7892–7927
Gomillion C T, Burg K J L. Stem cells and adipose tissue engineering. Biomaterials, 2006, 27(36): 6052–6063
Salcedo L, Sopko N, Jiang H H, et al. Chemokine upregulation in response to anal sphincter and pudendal nerve injury: potential signals for stem cell homing. International Journal of Colorectal Disease, 2011, 26(12): 1577–1581
Ko I K, Lee S J, Atala A, et al. In situ tissue regeneration through host stem cell recruitment. Experimental & Molecular Medicine, 2013, 45(11): e57
Zhou B, Han Z C, Poon M C, et al. Mesenchymal stem/stromal cells (MSC) transfected with stromal derived factor 1 (SDF-1) for therapeutic neovascularization: enhancement of cell recruitment and entrapment. Medical Hypotheses, 2007, 68(6): 1268–1271
Butler J M, Guthrie S M, Koc M, et al. SDF-1 is both necessary and sufficient to promote proliferative retinopathy. The Journal of Clinical Investigation, 2005, 115(1): 86–93
Zernecke A, Schober A, Bot I, et al. SDF-1a/CXCR4 axis is instrumental in neointimal hyperplasia and recruitment of smooth muscle progenitor cells. Circulation Research, 2005, 96(7): 784–791
Thevenot P, Nair A, Shen J, et al. The effect of incorporation of SDF-1a into PLGA scaffolds on stem cell recruitment and the inflammatory response. Biomaterials, 2010, 31(14): 3997–4008
Riccardo L, Planell J A, Mateos-Timoneda M A, et al. Role of ECM/peptide coatings on SDF-1a triggered mesenchymal stromal cell migration from microcarriers for cell therapy. Acta Biomaterialia, 2015, 18: 59–67
Nakamura T, Nishizawa T, Hagiya M, et al. Molecular cloning and expression of human hepatocyte growth factor. Nature, 1989, 342(6248): 440–443
Patel M B, Pothula S P, Xu Z, et al. The role of the hepatocyte growth factor/c-MET pathway in pancreatic stellate cell-endothelial cell interactions: anti-angiogenic implications in pancreatic cancer. Carcinogenesis, 2014, 35(8): S9
Neuss S, Becher E, Wöltje M, et al. Functional expression of HGF and HGF receptor/c-met in adult human mesenchymal stem cells suggests a role in cell mobilization, tissue repair, and wound healing. Stem Cells, 2004, 22(3): 405–414
Schenk S, Mal N, Finan A, et al. Monocyte chemotactic protein-3 is a myocardial mesenchymal stem cell homing factor. Stem Cells, 2007, 25(1): 245–251
De Becker A, Van Hummelen P, Bakkus M, et al. Migration of culture-expanded human mesenchymal stem cells through bone marrow endothelium is regulated by matrix metalloproteinase-2 and tissue inhibitor of metalloproteinase-3. Haematologica, 2007, 92(4): 440–449
Border W A, Noble N A. Transforming growth factor ß in tissue fibrosis. The New England Journal of Medicine, 1994, 331(19): 1286–1292
Huang Q, Goh J C, Hutmacher D W, et al. In vivo mesenchymal cell recruitment by a scaffold loaded with transforming growth factor ß1 and the potential for in situ chondrogenesis. Tissue Engineering, 2002, 8(3): 469–482
Ferrara N, Davis-Smyth T. The biology of vascular endothelial growth factor. Endocrine Reviews, 1997, 18(1): 4–25
Aiello L P, Avery R L, Arrigg P G, et al. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. The New England Journal of Medicine, 1994, 331(22): 1480–1487
Elçin Y M, Dixit V, Gitnick G. Extensive In vivo angiogenesis following controlled release of human vascular endothelial cell growth factor: implications for tissue engineering and wound healing. Artificial Organs, 2001, 25(7): 558–565
Kim S H, Hur W, Kim J E, et al. Self-assembling peptide nanofibers coupled with neuropeptide substance P for bone tissue engineering. Tissue Engineering Part A, 2015, 21(7–8): 1237–1246
Zhao L, Weir M D, Xu H H K. An injectable calcium phosphatealginate hydrogel-umbilical cord mesenchymal stem cell paste for bone tissue engineering. Biomaterials, 2010, 31(25): 6502–6510
Olmos Buitrago J, Perez R A, El-Fiqi A, et al. Core–shell fibrous stem cell carriers incorporating osteogenic nanoparticulate cues for bone tissue engineering. Acta Biomaterialia, 2015, 28: 183–192
Yilgor P, Sousa R A, Reis R L, et al. 3D plotted PCL scaffolds for stem cell based bone tissue engineering. Macromolecular Symposia, 2008, 269(1): 92–99
Ye C, Hu P, Ma M X, et al. PHB/PHBHHx scaffolds and human adipose-derived stem cells for cartilage tissue engineering. Biomaterials, 2009, 30(26): 4401–4406
Lee C H, Cook J L, Mendelson A, et al. Regeneration of the articular surface of the rabbit synovial joint by cell homing: a proof of concept study. Lancet, 2010, 376(9739): 440–448
Erggelet C, Endres M, Neumann K, et al. Formation of cartilage repair tissue in articular cartilage defects pretreated with microfracture and covered with cell-free polymer-based implants. Journal of Orthopaedic Research, 2009, 27(10): 1353–1360
Wang A, Tang Z, Park I H, et al. Induced pluripotent stem cells for neural tissue engineering. Biomaterials, 2011, 32(22): 5023–5032
Zhuang Y M, Huojia M, Xu H, et al. Effects of transforming growth factor-ß_3 and dental pulp stem cells in repairing rabbit facial nerve injury. Journal of Chinese Practical Diagnosis and Therapy, 2015, (7) (in Chinese)
Zhu T, Tang Q, Shen Y, et al. An acellular cerebellar biological scaffold: Preparation, characterization, biocompatibility and effects on neural stem cells. Brain Research Bulletin, 2015, 113: 48–57
Jin G, Prabhakaran M P, Ramakrishna S. Stem cell differentiation to epidermal lineages on electrospun nanofibrous substrates for skin tissue engineering. Acta Biomaterialia, 2011, 7(8): 3113–3122
Healy K E, Guldberg R E. Bone tissue engineering. Journal of Musculoskeletal & Neuronal Interactions, 2007, 7(4): 328–330
Barnes B, Boden S D, Louis-Ugbo J, et al. Lower dose of rhBMP- 2 achieves spine fusion when combined with an osteoconductive bulking agent in non-human primates. Spine, 2005, 30(10): 1127–1133
Goekoop-Ruiterman Y P M, de Vries-Bouwstra J K, Allaart C F, et al. Clinical and radiographic outcomes of four different treatment strategies in patients with early rheumatoid arthritis (the Best study): a randomized, controlled trial. Arthritis and Rheumatology, 2005, 52(11): 3381–3390
Ko I K, Lee S J, Atala A, et al. In situ tissue regeneration through host stem cell recruitment. Experimental & Molecular Medicine, 2013, 45(11): e57
Sirko S, Neitz A, Mittmann T, et al. Focal laser-lesions activate an endogenous population of neural stem/progenitor cells in the adult visual cortex. Brain, 2009, 132(8): 2252–2264
Jayarama Reddy V, Radhakrishnan S, Ravichandran R, et al. Nanofibrous structured biomimetic strategies for skin tissue regeneration. Wound Repair and Regeneration, 2013, 21(1): 1–16
Kamel R A, Ong J F, Eriksson E, et al. Tissue engineering of skin. Journal of the American College of Surgeons, 2013, 217(3): 533–555
Ma K, Liao S, He L, et al. Effects of nanofiber/stem cell composite on wound healing in acute full-thickness skin wounds. Tissue Engineering Part A, 2011, 17(9–10): 1413–1424
Acknowledgement
This research was supported by the National Natural Science Foundation of China (Grant No. 81371979).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yu, Y., Sun, B., Yi, C. et al. Stem cell homing-based tissue engineering using bioactive materials. Front. Mater. Sci. 11, 93–105 (2017). https://doi.org/10.1007/s11706-017-0373-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11706-017-0373-0