Abstract
A new difference spectrophotometric method for the determination of prothipendyl hydrochloride in commercial pharmaceutical preparations has been proposed. The method includes oxidation of an aliquot of the drug solution with potassium caroate to form the corresponding sulfoxide (ε = (5.83 ± 0.07) × 103 L/mol cm) and subsequent measurement of the absorbance at 340 nm compared to that of the unoxidized drug solution of equal concentration. The graph of Beer’s law for prothipendyl hydrochloride shows that the ΔA values measured at the corresponding wavelength are proportional to the concentration of the drug in the concentration range of 1.5–70 µg/mL. The characteristics of the curve calibration curve of the linear regression equation were as follows: ΔA = (69.24 ± 1.78) × 10–4 C (where C in μg/mL). A new spectrophotometric technique has been developed and the possibility of quantitative determination of prothipendyl hydrochloride monohydrate in Dominal® tablets of 40 mg has been demonstrated. RSD ≤ 1.3%; (│δ│ < tα × RSD/\(\sqrt{n}\)), where µ is quantification data given in the Certificate of Quality, δ = (x̅-µ) 100%/µ. The resulting difference in absorption does not depend on the presence of excipients and possible API oxidation products in the composition.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
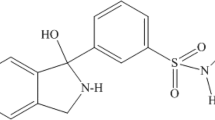
Prothipendyl (PTP), available under brand names such as Dominal, Timovan, and Tolnate, is also known as azaphenothiazine or frenotropin. Belonging to the azaphenothiazine category, it functions as an anxiolytic, antiemetic, and antihistamine. Widely distributed in Europe, this compound is utilized to address anxiety and agitation associated with psychotic syndromes (Apotheker-Verein 2000; Elks 2014; Leigh et al. 2012; Reynolds 1982). It distinguishes itself from promazine solely through the substitution of a single carbon atom with a nitrogen atom within the tricyclic ring system (Fig. 1).
In pharmaceuticals, the prothipendyl hydrochloride-1-water (INN) salt is used. It is a pastel yellow to greenish yellow odorless crystalline powder with the formula C16H19N3S·HCl·H2O and a melting point of 108 to 112 °C (as anhydrous 177 to 178 °C) (Greenstein 2007).
Due to their valuable therapeutic and pharmacological properties, phenothiazine and azaphenothiazine derivatives are the subject of many scientific studies. A review of the literature shows that methods for the determination of phenothiazine and azaphenothiazine derivatives mainly include chromatographic (Debailleul et al. 1991; Diehl and Karst 2000; Krämer et al. 2018; Kumazawa et al. 2011) and spectrophotometric (Blazheyevskiy 2019; Misiuk and Kleszczewska 2001; Nascentes et al. 2002).
The vital importance of this drug has prompted the development of many analytical methods for its determination. In addition to official methods based on non-aqueous titration (acidimetry) and direct UV spectrophotometry, various spectrophotometric methods based on oxidation reactions with the formation of intensely colored radical cations have also been used (Misiuk and Kleszczewska 2001; Puzanowska-Tarasiewicz et al. 2005). However, it is known that the color stability of the radical cation (3a-3b) (Fig. 2) depends mainly on the oxidizing agent used and the acidity of the medium. In the case of a strong oxidizing agent, the color of the radical quickly disappears due to the second step of the reaction leading to the formation of a colorless sulfoxide (2) (Fig. 2). This effect can lead to decreased assay sensitivity and reproducibility. Also, some of these methods have some disadvantages such as a high concentration of acid solution (analysis environment) and others do not have high enough sensitivity and require a very long heating time (Misiuk and Kleszczewska 2001; Puzanowska-Tarasiewicz et al. 2005).
Phenothiazine drugs are currently manufactured in various dosage forms, either as a single drug or in combination with one or more other drugs. Simple spectrophotometric methods, such as those used by the British Pharmacopoeia, typically involve extracting or diluting the drug, followed by measurement of ultraviolet absorbance. These procedures lack specificity and may be affected by other ultraviolet-absorbing drugs, dyes and flavors, or oxidation products of phenothiazine drugs, which are the corresponding phenothiazine sulfoxide and sulfone (Krämer et al. 2017, 2018).
An indirect difference spectrophotometric method for the analysis of phenothiazine preparations in various commercial preparations is described. The assay can be performed as rapidly as the direct spectrophotometric method and is specific for an intact phenothiazine preparation. The method includes the oxidation of an aliquot of the drug solution with peroxyacetic acid (preliminarily obtained as a result of the reaction of hydrogen peroxide and acetic acid) with the formation of phenothiazine sulfoxide and the measurement of the optical density of the solution in the range of 340–360 nm using an unoxidized drug solution equal to the concentration in the reference cuvette. The resulting absorption difference is proportional to the intact phenothiazine drug and is independent of the presence of excipients, degradation products, or other drugs in the formulation. Provided that these substances have not changed under the action of the oxidizing agent, their concentration in the test and reference solutions is the same, and their absorption difference is zero (Davidson 1976).
However, peroxyacetic acid is an unstable compound, and its solution is an equilibrium mixture of hydrogen peroxide, acetic acid and, in fact, peroxyacetic acid in water. In addition, the peroxyacetic acid solution has a strong irritating malodor. Selective chromatographic methods (Debailleul et al. 1991; Diehl and Karst 2000) are sensitive enough to detect normally low therapeutic levels of prothipendyl in biological fluids. These methods are useful when the sample matrix is quite complex and the drug concentration is low. In pharmaceutical analysis, where analyte concentration levels are quite high, the main goal is to develop fast, simple, reproducible and inexpensive methods that can easily find application in routine analyses and quality control laboratories.
Spectrophotometry, due to its simplicity, is very useful for the quantitative analysis of drugs in pharmaceuticals. The number of spectrophotometric methods for determining the cited drug is very limited.
The application of difference spectrophotometry is very advantageous both in qualitative and quantitative analysis. However, the real great importance of difference spectrophotometry is in quantitative analysis as a consequence of its potential to increase the selectivity of the measurement (Gorog 1995).
So, the authors set the task to develop a new and simple method for prothipendyl hydrochloride determination. It seems promising to carry out the analysis of prothipendyl hydrochloride in the form of the corresponding stable S-oxide, which is easily obtained in a slightly acidic medium using aliphatic diperoxyacids or inorganic Caro peroxoacid (Blazheyevskiy 2019; Blazheyevskiy 2017a, b; Davidson 1976).
Experimental
Materials
Chemicals
Solutions of prothipendyl hydrochloride (LGC, Luckenwalde, Germany) and prothipendyl sulfoxide (Toronto Research Chemicals Inc.) were prepared by dissolving appropriate weighed amounts of the substances in water and a water–ethanol solution 1:1 (v/v), respectively. All other used chemicals were analytical grade. All excipients were of pharmacopoeial purity.
Dominal® 40 mg (TEVA GmbH) coated tablets, 600093498; E 307829.01-Z03; Identifier (PZL)—14179534; (Ch. B.) 0602521. Active ingredient: prothipendyl hydrochloride 1H2O (each film-coated tablet contains 40 mg of the active substance). Excipients: cellulose microcrystalline, lactose monohydrate 39.40 mg; corn starch; magnesium stearate; silica colloidal; talc; sucrose 55.43 mg; quinoline yellow aluminum salt/indigo carmine aluminum salt (7:3); titanium dioxide; macrogol 35000; calcium carbonate; carmellose sodium; povidone K25; polysorbate 20; mountain glycol wax. The average tablet weight is 0.29979 g.
Potassium caroate (KHSO5), also known as potassium peroxymonosulfate, potassium monopersulfate, is a white powder and chlorine-free oxidizer. It is a strong oxidizing agent with an oxidizing potential similar to that of chlorine. The triple salt 2KHSO5·KHSO4·K2SO4 (known under the trade name Oxone, Acros Organics) is a higher stability form. The active ingredient in Oxone® is KHSO5.
Preparation of KHSO5 solution, 0.02 mol/L. About 0.7 g of Oxone was dissolved in 70 mL of double-distilled water in a 100 mL volumetric flask, made up to the mark with water and mixed thoroughly.
Preparation of KHSO5 solution, 0.005 mol/L. A portion of about 0.175 g of Oxone was dissolved in 100 mL of double-distilled water.
The exact KHSO5 content was determined by iodometric titration (Blazheyevskiy 2017a).
Preparation of 0.01 M sulfuric acid solution. The solution was prepared from the standard titer fixanal in a 1000 mL volumetric flask.
Preparation of a solution of working reference standard sample (WSS) of prothipendyl hydrochloride monohydrate, 0.40 mg/mL (335.9 μg/mL in terms of prothipendyl base). About 40 mg (accurately weighed) of a standard sample of prothipendyl hydrochloride monohydrate is placed in a 100 mL volumetric flask, dissolved in 70 mL of a 0.01 mol/L sulfate acid solution, and the volume of the solution is adjusted with the same solvent to obtain a solution containing about 0.03359% w/v (or 335.9 µg/mL) prothipendyl base.
Method for the quantitative determination of prothipendyl hydrochloride content in coated tablets, 40 mg (TEVA GmbH).
Add 50–70 mL of 0.01 mol/L sulfuric acid solution to a weighed portion of powdered tablets equal to the average weight of the tablet, mix with ultrasound for 10 min, dilute with 0.01 mol/L sulfuric acid solution to 100 mL and filter through a paper filter with blue ribbon to obtain a clear solution containing about 0.03359% w/v (or 335.9 µg/mL) prothipendyl base (solution A).
Dilute 10.00 mL of solution A to 100 mL with 0.01 mol/L sulfuric acid solution (solution B). The solution is photometered at an analytical wavelength of 340 nm in a 5 cm quartz cuvette against a 0.01 mol/L sulfuric acid solution as a compensation solution.
To another 10 mL of solution A add 10.00 mL of 0.002 mol/L KHSO5 solution, mix and dilute with 0.01 mol/L sulfuric acid solution to 100 mL and leave for 5 min (solution C). The solution is photometered at an analytical wavelength of 340 nm in a 5 cm quartz cuvette against a KHSO5 solution and sulfuric acid of the same concentration as in solution C as the compensation solution.
A procedure was repeated using a solution of RS prothipendyl hydrochloride monohydrate in 0.01 mol/L sulfuric acid (look preparation of a solution of working standard sample of prothipendyl hydrochloride monohydrate, 0.40 mg/mL (335.9 μg/mL in terms of prothipendyl base)) instead of solution A, starting with “Dilute 10.00 mL of solution A to 100 mL…” and calculate the C16H19N3S·HCl·H2O content using the reported C16H19N3S·HCl·H2O content in RS prothipendyl hydrochloride monohydrate.
The prothipendyl hydrochloride monohydrate C16H19N3S·HCl·H2O content in the preparation as a percentage of the declared amount (X) is calculated by the formula:
where ΔA1 is difference between absorbances of the test solution and prothipendyl hydrochloride monohydrate solution (solution C versus solution B);
ΔA0 is the difference between the absorbances of the RS prothipendyl hydrochloride monohydrate test solution (solution C) and the RS prothipendyl hydrochloride monohydrate (solution B) (RS solution C versus RS solution B);
a1 is weighed powder of crushed tablets, mg;
a0 is weight of a standard sample of RS prothipendyl hydrochloride monohydrate, mg;
P is the content of the main substance in the standard sample prothipendyl hydrochloride monohydrate, %;
G is average weight of one tablet, mg;
L is the declared amount of prothipendyl hydrochloride monohydrate in one tablet, mg.
Instruments and measurements
A double-beam Shimadzu UV–Visible spectrophotometer, with spectral bandwidth of 1 nm wavelength accuracy ± 0.5 nm, Model – UV 1800 (Japan), Software UV-Probe 2.62, and a pair of 1 cm matched quartz cells, and also in a cuvette with an absorbing layer thickness of 5 cm on a Specol 11 spectrophotometer (Carl Zeiss Jena) with an EK 5 attachment were used to measure absorbance of the resulting solutions.
The product formed by the reaction of prothipendyl hydrochloride with an oxidizing agent under analytical conditions was confirmed to be prothipendyl sulfoxide by comparing its ultraviolet absorption spectrum with that of an authentic sample of prothipendyl sulfoxide (PTPO). Maximum and minimum absorption bands of the product were at λmax = 207, 239, 276, 340 nm and λmin = 219, 258, 309 nm, respectively (De Leenheer 1973).
Thin-layer chromatographic properties of tested compounds were investigated on TLC aluminum plates (10 × 15 cm) coated with 8–12 μm silica gel, 254 nm PTSH-AF-V-UF (Sorbfil). The extract of the oxidation product was evaporated at room temperature collected and identified with TLC using chloroform–methanol–ammonia (60:10:0.5) as a developing system followed by treatment of the adsorption zones with Wagner’s reagent. The oxidation product was well resolved from the pure drug with significantly different Rf values (PTP, Rf = 0.74 ± 0.02; PTPO, Rf = 0.58 ± 0.01).
Other used instruments: Analytical Balance RAD WAG AS 200/C, pH–meter I-160MI and IKA orbital shaker KS4000i.
Results and discussion
We have found that the drug can be determined by an indirect difference spectrophotometric method based on the absorption of the sulfoxide derivative of the drug in relation to the absorption of the non-oxidized drug solution. The sulfoxide derivative is formed quickly and quantitatively at room temperature by adding a solution of KHSO5 in the form of Oxone, which is a ternary compound 2KHSO5·KHSO4·K2SO4. Oxone has a longer shelf life than potassium peroxomonosulfate (Crandall et al. 2012). A white, water-soluble solid, Oxon loses < 1% of its oxidizing power within a month. The standard electrode potential for potassium peroxomonosulfate is + 1.81 V with a half-reaction to form hydrosulfate (pH = 0) (Spiro 1979).
As a result of the S-oxide formation, an intense bathochromic shift of the spectrum of the analyte takes place. The UV absorption spectra of the PTP, PTPO and KHSO5 are shown in Fig. 3. As seen, the PTPO showed an absorbance at 340 nm, while the absorbance of PTP at that wavelength was lower. The 340 nm wavelength was chosen to follow and determine the reaction kinetics, taking into account the absorbance of PTP at that wavelength. KHSO5 showed almost no absorbance in the UV region and thus did not interfere with any UV measurements. An increase in the wavelength of maximum absorbance was realized with enhanced specificity.
Method for constructing a calibration graph. With the help of a microburette, sequentially measured 0.50; 1.00; 1.50; 2.00; 2.50; 3.00; 3.50; 4.00; 4.50 mL of a solution of WSS. Prothipendyl hydrochloride monohydrate in volumetric flasks of 25 mL, add to each successively 0.50; 1.00; 1.50; 2.00; 2.50; 3.00; 3.50; 4.00; 4.50 mL of a 0.002 mol/L KHSO5 solution, shake well and dilute the volume of the solution with 0.01 mol/L sulfuric acid, stopper the flask and mix thoroughly. The solutions are photometered at an analytical wavelength of 340 nm in a 1 cm (in a 5 cm also) quartz cuvette against a potassium caroate and 0.01 mol/L sulfuric acid solution as a compensation solution.
The extinction coefficient found as the slope of the absorbance versus concentration plot or the molar absorbance ε (L/mol cm) at λmax = 340 nm for PTPO in 0.01 mol/L H2SO4 solution (slope of absorbance on PTPO concentration is (5.83 ± 0.07) × 103 L mol /cm (Figs. 4 and 5).
The graph of Beer’s law for prothipendyl hydrochloride shows that the ΔA values measured at the corresponding wavelength of maximum absorption difference are proportional to the concentration of the drug in oxidized and non-oxidized solutions in the concentration range of 0–0.007% (m/v) (Fig. 6).
The characteristics of the curve calibration curve of the linear regression equation were as follows: ΔA = (69.24 ± 1.78) × 10–4 C + (0.356 ± 8.04) × 10–3 (where C in μg/mL) (Table 1).
The UV spectrophotometric determination of sulfoxide proved to be a sufficiently reliable and sensitive method. The developed method of quantitative determination makes it possible to determine prothipendyl hydrochloride monohydrate in the concentration range of 5–70 μg/mL. The limit of detection, LOD (3S), is 1.5 µg/mL.
Specificity
A number of substances that may be present in dosage forms of PTP, either in the form of degradation products or in the composition of drugs as auxiliary, were studied under analysis conditions for an optical density difference of about 340–342 nm. The following substances give a zero difference in absorption and therefore do not interfere with the analysis: PTPO and excipients in generally accepted prescribed amounts: microcrystalline cellulose, lactose monohydrate, corn starch, magnesium stearate, colloidal silica gel, talc, sucrose, quinoline yellow, quinoline aluminum salt yellow and aluminum salt of indigo carmine (7:3), titanium dioxide, macrogol 35,000, calcium carbonate, carmellose sodium, povidone K25, polysorbate 20.
The difference in the absorption of solutions is proportional to the concentration of the azaphenothiazine preparation in the preparation and is specific for the intact preparation in the presence of oxidative and photochemical decomposition products, dyes and other tablet excipients.
A new spectrophotometric technique has been developed and the possibility of quantitative determination of prothipendyl hydrochloride monohydrate in Dominal® tablets of 40 mg has been demonstrated. RSD ≤ 1.3%; (│\(\delta \)│ < tα × RSD/\(\sqrt{n}\)), where µ is quantification data given in the Certificate of Quality (Table 2). δ = (x̅−µ)100 %/µ.
Conclusions
The approach has been formulated, enabling the new potential for quantitatively analyzing prothipendyl hydrochloride in pharmaceutical preparations. The method includes oxidation of the drug solution with potassium caroate in the form of Oxone to form the corresponding sulfoxide and subsequent measurement of the absorbance at 340 nm compared to that of the unoxidized drug solution of equal concentration. The product developed by the reaction of prothipendyl hydrochloride with an oxidizing agent under analytical conditions was confirmed to be prothipendyl sulfoxide by comparing its ultraviolet absorption spectrum with that of an authentic sample of prothipendyl sulfoxide. The difference in the absorption of solutions is proportional to the concentration of the prothipendyl hydrochloride in the range of 1.5–70 µg/mL. It is specific for the intact preparation in the presence of excipients, oxidative and photochemical decomposition products, and dyes. A new spectrophotometric technique has been developed and the possibility of quantitative determination of prothipendyl hydrochloride monohydrate in Dominal® tablets of 40 mg has been demonstrated. The outcomes indicate that the technique exhibits good precision and accuracy, demonstrating an acceptable agreement with the results derived from the official method. The received relative standard deviation (RSD) is limited to ≤ 1.3% (δ = –0.62%). The limit of detection (LOD) and limit of quantitation (LOQ) values were 1.5 μg/ml and 4.9 μg/ml for prothipendyl hydrochloride, respectively.
References
Apotheker-Verein, S (2000) Index Nominum 2000: international drug directory, vol 17. Taylor & Francis
Blazheyevskiy M (2017a) Application of derivatization by means of peroxy acid oxidation and perhydrolysis reactions in pharmaceutical analysis. Monograph. Ivan Franko National University of Lviv, Lviv
Blazheyevskiy M (2017b) Application derivatization by means perhydrolysis reactions in pharmaceutical analysis. Methods Objects Chem Anal 12(1):31–54. https://doi.org/10.17721/moca.2017.31-54
Blazheyevskiy M (2019) Spectrophotometric and spectrofluorimetric determination of the 2-and 10-disubstituted phenothiazines using peroxy acid oxidation. Curr Top Anal Chem 11:67–80
Crandall J, Shi Y, Burke C, Buckley B (2012) Potassium Monoperoxysulfate. In: Encyclopedia of Reagents for Organic Synthesis (EROS). https://doi.org/10.1002/047084289X.rp246.pub3
Davidson A (1976) The determination of phenothiazine drugs in pharmaceutical preparations by a difference spectrophotometric method. J Pharm Pharmacol 28(11):795–800. https://doi.org/10.1111/j.2042-7158.1976.tb04059.x
De Leenheer A (1973) Ultraviolet spectrophotometry of phenothiazine derivatives and analogs. J Assoc off Anal Chem 56(1):105–118
Debailleul G, Khalil A, Lheureux Ph (1991) HPLC quantification of zolpidem and prothipendyl in a voluntary intoxication. J Anal Toxicol 15(1):35–37. https://doi.org/10.1093/jat/15.1.35
Diehl G, Karst U (2000) Post-column oxidative derivatization for the liquid chromatographic determination of phenothiazines. J Chromatogr A 890(2):281–287. https://doi.org/10.1016/s0021-9673(00)00607-5
Elks J (2014) The dictionary of drugs: chemical data: chemical data, structures and bibliographies. Springer. ISBN 978-1-4757-2085-3
Gorog S (1995) Ultraviolet-visible spectrophotometry in pharmaceutical analysis. CRC Press
Greenstein G (2007) The Merck index: an encyclopedia of chemicals, drugs, and biologicals. Ref Rev 21(6):40. URL: https://echa.europa.eu/web/guest/legal-notice
Krämer M, Broecker S, Madea B, Hess C (2017) Confirmation of metabolites of the neuroleptic drug prothipendyl using human liver microsomes, specific CYP enzymes and authentic forensic samples - Benefit for routine drug testing. J Pharm Biomed Anal 145:517–524. https://doi.org/10.1016/j.jpba.2017.07.011
Krämer M, Heese P, Banger M, Madea B, Hess C (2018) Range of therapeutic prothipendyl and prothipendyl sulfoxide concentrations in clinical blood samples. Drug Test Anal 10(6):1009–1016. https://doi.org/10.1002/dta.2319
Kumazawa T, Hasegawa C, Uchigasaki S, Lee XP, Suzuki O, Sato K (2011) Quantitative determination of phenothiazine derivatives in human plasma using monolithic silica solid-phase extraction tips and gas chromatography-mass spectrometry. J Chromatogr A 1218(18):2521–2527. https://doi.org/10.1016/j.chroma.2011.02.070
Leigh D, Pare C, Marks J (2012) A concise encyclopaedia of psychiatry. Springer Science & Business Media. ISBN 978-94-011-5913-5
Misiuk W, Kleszczewska E (2001) Application of ammonium peroxidisulfate and metavanadate for spectrophotometric determination of prothipendyl hydrochloride. Acta Pol Pharm 58(2):87–92
Nascentes C, Cárdenas S, Gallego M, Valcárcel M (2002) Continuous photometric method for the screening of human urines for phenothiazines. Anal Chim Acta 462(2):275–281. https://doi.org/10.1016/S0003-2670(02)00317-3
Puzanowska-Tarasiewicz H, Kužmicka L, Karpińska J, Mielech-Łukasiewicz K (2005) Efficient oxidizing agents for determination of 2, 10-disubstituted phenothiazines. Anal Sci 21(10):1149–1153. https://doi.org/10.2116/analsci.21.1149
Reynolds J (1982) Martindale: the extra pharmacopoeia. The Pharmaceutical Press, London
Spiro M (1979) The standard potential of the peroxosulphate/sulphate couple. Electrochim Acta 24(3):313–314. https://doi.org/10.1016/0013-4686(79)85051-3
Funding
No funding was received.
Author information
Authors and Affiliations
Contributions
LK and TK conducted the experiments, OM and MB analyzed data. VM and OS assisted in the experiments and discussed the results. MB and LK wrote the manuscript and drew the graphs. OM, VM and OS revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Ethical approval and consent to participate
No applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mozgova, O., Blazheyevskiy, M., Kryskiw, L. et al. Determination of prothipendyl by difference spectrophotometric method based on the absorption of its sulfoxide. Chem. Pap. 78, 2613–2619 (2024). https://doi.org/10.1007/s11696-023-03266-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11696-023-03266-5