Abstract
Three green and facile spectrophotometric methods were developed for the assay of Petro® components; drotaverine HCl (DRT), caffeine (CAFF), and paracetamol (PAR). The three methods depend on measuring the absorbance of the studied drugs through their ethanolic solution. The first derivative spectrophotometry (FDS) at (Δλ = 10) were good parameters for DRT and CAFF resolution; DRT and CAFF could be well calibrated using FDS at 320 and 285 nm, respectively. PAR could be estimated at 308 nm utilizing the second derivative spectrophotometry (SDS). Method II relies on the double divisor ratio derivative spectroscopy (DDRDS). The first derivative was applied on each drug where they would be assayed at 309, 288, and 255 nm for DRT, CAFF, and PAR, respectively. Method III depends on the mean centering (MCR) technique. DRT, CAFF, and PAR could be determined at 309, 214, and 248 nm, respectively. The concentrations were rectilinear in the ranges of 2–20 µg/mL for DRT, 1.5–15 µg/mL for CAFF, and 2–40 µg/mL for PAR in double devisor and mean centering but PAR from 5 to 40 µg/mL in derivative method. Method validation was performed according to ICH guidelines assured by the agreement with the comparison method. In addition, greenness assessment of the proposed methods was investigated. The application of the proposed method was extended to analyse tablet dosage form and performing invitro dissolution testing.
Similar content being viewed by others
Introduction
Ultraviolet spectrophotometry is among the most convenient and useful quantitative and qualitative methods, especially in multicomponent analysis [1] by minimizing the cumbersome task of separating interferents [2]. Derivative spectrophotometry is a useful analytical method For gathering variable data from the spectra of overlapped bands and minimizing the effects of baseline shifts and tilts. It entails computing and plotting one of the spectral curves’ and mathematical derivatives.Consequently, a spectrum’s information content is presented in a manner that may be more useful [3]. The basis of double divisor ratio derivative spectroscopy (DDRDS) is the derivative of the ratio spectrum, which is produced by dividing the ternary mixture's absorption spectra by a standard spectrum of a mixture of two of the three compunds in the desired mixture. Utilizing the calibration graphs for each compound, which are obtained by measuring the amplitude chosen, the concentrations of the three compounds in their mixture are identified [4]. One of the newest and most effective spectrophotometric techniques for quantitative analysis of multicomponent mixtures, mean centering of ratio spectra (MCR), does not require any derivatization stages. Compared to chromatography, this procedure is recognized to be more efficient in terms of time and cost [5].
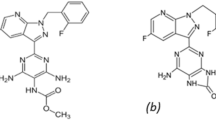
Drotaverine HCl (DRT), Caffeine (CAFF) and Paracetamol (PAR) are official in British pharmacopeia [7] and United States pharmacopeia [8]. Drotaverine HCl (DRT) is 1-[(3,4-diethoxyphenyl)methylene]-6,7-diethoxy-1,2,3,4-tetrahydroisoquinoline hydrochloride [7]. DRT is antispasmodic drug [9]. Numerous analytical methods assessed DRT in different matrices. Recent articles regarding DRT analysis like UV visible spectrophotometry [10,11,12,13], spectrofluorimetric methods [14], electrochemical methods [15,16,17,18], potentiometric titration methods [19], chromatographic methods [20,21,22,23,24,25,26,27,28,29,30,31,32,33]. Caffeine (CAFF) is 1,3,7-trimethyl 3,7 dihydro-1 h- purine 2,6-dione [7]. The advantages include a decrease in tiredness and weariness and an improvement in mood. It has various pharmacological effects, including raising gastric output, fostering lipolysis, boosting skeletal and muscular contraction, and raising diuresis. Long-term sleeplessness, peptic ulcers, and elevated serum cholesterol are the main negative consequences of caffeine [34]. Numerous analytical methods were used for the assessment of CAFF in variable matrices like UV/Visible spectrophotometric methods like [35,36,37,38,39], partial least‐squares algorithm (PLS) [40], chromatographic methods [41,42,43,44,45,46,47,48,49,50,51,52] and electrochemical methods [53,54,55,56]. Paracetamol (PAR) is N-(4-hydroxyphenyl) acetamide [6]. PAR has analgesic and antipyretic properties and anti-inflammatory activity [9]. Numerous analytical methods were used for the assessment of PAR in various matrices like UV/Visible spectrophotometric methods [57,58,59,60,61], spectrofluorimetric methods [62], electrochemical methods [63,64,65,66,67,68,69], chromatographic methods [70,71,72,73,74] and other methods like flow injection analysis [75], coupling of sequential injection analysis (SIA) and fluorometric solid phase transduction [76] and capillary zone electrophoresis [77]. The concurrent estimation of the studied medications was achieved by using spectrophotometric and HPTLC methods [33], sweeping-micellar electrokinetic chromatography [78], and RP-HPLC method [79].
DRT, CAFF, and PAR are co-formulated in one tablet dosage form under trade names Petro® tablets containing 40 mg DRT, 60 mg CAFF, and 400 mg PAR. The pharmaceutical ratio of co-formulations was found to be 1:1.5:10 (w/w) for DRT: CAFF: PAR, respectively.
This work aims to represent new univariate spectrophotometric methods to evaluate the assay of DRT, CAFF, and PAR co-formulated in Petro® tablets in a pharmaceutical ratio 1:1.5:10 (w/w).
Experimental
Apparatus and software
-
UV-PC spectrophotometer (Shimadzu 1650), supplied with 1.0 cm quartz cells.
-
An ultrasonic bath (model SS 101 H 230, USA) was used for sonication.
-
Matlab R2022, an (8.2.0.701) software, was used for performing the wholly chemometric procedures. PLS Toolbox software, version 2.1, was used to carry out mean centering through our own written codes in Matlab software. MCR was performed using PLS toolbox software version 2.1.
Materials and solvents
-
Reference standard samples of DRT, CAFF, and PAR were purchased from Amoun Pharmaceutical Company in El-Obour City, Egypt. These samples were confirmed to have purity levels of 99.5, 99.5, and 99.4%, respectively.
-
Petro® tablets; 40 DRT, 60 CAFF, and 400 mg PAR per tablet, a product of Alphamoun Pharmaceuticals Co., industrial zone, Badr City, Egypt (batch No. 12101299), purchased from a local pharmacy in Egypt.
-
The inactive ingredients other than DRT, CAFF and PAR in Petro tablet (Palcebo): magnesium stearate, lactose monohydrate, maize starch, calcium hydrogen phosphate dihydrate and talc were obtained from pharmaceutical chemistry department,faculty of pharmacy, delta university for science and technology.
-
Filtered deionized water was used throughout the work.
-
Ethanol, methanol, acetonitrile, propanol, and acetone were HPLC grade, were obtained from Fisher, UK.
Preparation of standard solution
By dissolving 0.01 g of each drug in 100 mL of ethanol, standard stock solutions containing (100 µg/mL) of each investigated medicines were created. The working solutions were produced from the standard stock solutions by employing the serial dilution procedure with the same solvent for DRT, CAFF, and PAR.
Procedures
Spectral features
From 200 to 400 nm, the absorption spectra of ethanolic solutions containing various DRT, CAFF, and PAR concentrations were scanned.
Calibration graphs development
-
–Derivative method
Accurately measured volumes of DRT, CAFF and PAR standard solutions were transferred into separate sets of 10 mL volumetric flasks to get concentrations in the range of 2–20 µg/mL for DRT, 1.5–15 µg/mL for CAFF, and 5–40 µg/mL for PAR in derivative method while 5–40 µg/mL, completed with ethanol to the mark. The absorption spectra of the prepared DRT, CAFF, and PAR solutions were recorded against ethanol as blank over 200–400 nm. The first derivative was then manipulated using scaling factor = 10.0, smoothing level (× 10), and Δλ = 10.0 nm. The trough amplitude was measured at 320 nm for DRT and 285 nm for CAFF. The SDS with Δλ = 10.0 nm, smoothing level (× 10), and scaling factor 20 is the optimum solution for PAR resolution at 307 nm. All drugs were measured and plotted against final concentration in μg/mL to develop a calibration graph. Alternatively, the regression equation was derived.
-
–Double divisor ratio derivative method
The ratio spectra were generated by recording the absorption spectra of the solutions produced at divergent concentrations of one of the drugs (DRT, CAFF, and PAR) and dividing them by the sum of the absorption spectra of the two other drugs. The ratio spectra of DRT were achieved by dividing DRT spectra against 9 µg/mL of CAFF and 8 µg/mL of PAR, called the double divisor for the ratio spectra of CAFF. CAFF absorption spectra were divided over 8 µg/mL DRT and 8 µg/mL PAR. Finally, For PAR ratio spectra, dividing PAR absorption spectra 6 µg/mL CAFF and 6 µg/mL DRT, the D1 of the ratio spectra were displayed with smoothing level(× 10) and scaling factor 10.
The concentrations of the studied drugs were estimated by measuring the amplitude at 309 nm for DRT, 288 nm for CAFF, and 255 nm for PAR and at, which matched the first derivative of the ratio spectra in the specified spectral region (200–400 nm). The amplitudes were measured and plotted against the final concentration in μg/mL to establish a calibration graph. Alternatively, the regression equation was derived.
-
–Mean Centering Method
For DRT: the recorded spectra were divided by the standard spectrum of 9 μg/mL CAFF and 8 μg/mL PAR to obtain the ratio spectra, which was then mean centered. Then the MCR was then obtained.
For CAFF, the recorded spectra were divided by 8 μg/mL DRT and 8 μg/mL PAR to obtain the first ratio spectra, which was then mean centered.
Similarly, the recorded spectra of PAR were divided by 6 μg/ml DRT and 6 μg/mL CAFF and the obtained ratio spectra were mean-centered.
The mean-centered values of the ratio spectra at 309, 214, and 248 nm for DRT, CAFF, and PAR, respectively, were measured and plotted against the corresponding concentration of each drug to construct their respective calibration graphs, then the regression equations were derived.
Determination of the studied drugs in synthetic mixtures
To generate a synthetic mixture of three variable concentrations within the required range, exact amounts of the working stock solutions of DRT, CAFF, and PAR were placed into 10 mL volumetric flasks.
Preparation of dosage form solutions (Petro® tablets)
Ten tablets were triturated and weighed accurately. One tablet containing 40 mg DRT, 60 mg CAFF, and 400 mg PAR included an exact weight of powder extracted with a specific amount of ethanol, sonicated for 30 min, finished to the correct weight with ethanol, and then filtered. More adequate dilutions were made to prepare the samples within the drugs’ concentration range.
Results and discussion
This study set out to estimate DRT, CAFF, and PAR (Fig. 1) in their ternary mixture using straightforward univariate methods. Since their UV-absorption spectra had a lot of overlap, as seen in Fig. 2, it was difficult to determine them directly. While the spectra of DRT, CAFF, and PAR could be easily resolved and calculated upon applying the proposed approaches.
Derivative technique (Method I)
A great overlapping was observed between the absorption spectra of DRT, CAFF, and PAR, which is absurd to be separated by conventional spectrophotometry (Fig. 2). So, the derivative technique is a good alternative for improving the selectivity. Divergent smoothing levels and scaling factor values were examined to enhance this mixture's resolution. It was found that first derivative spectrophotometry with scaling factor 20, Δ λ = 10, and smoothing level 10 were good parameters for DRT and CAFF resolution (Fig. 3a). DRT and CAFF first derivative absorption spectra were resolved, while for PAR resolution and separation; the second derivative with Δλ = 10.0 nm smoothing level (× 10) and scaling factor 20 was applied (Fig. 3b). DRT and CAFF could be well calibrated using FDS at 320 and 285 nm, respectively (Figs. 4, 5). PAR could be well calibrated using Second derivative spectrophotometry (SDS) at 307 nm (Fig. 6). These wavelengths were chosen as they are zero crossing points for the other drugs and have accurate and reproducible results.
Double divisor technique (Method II)
Figure 7 illustrates the ratio spectra of variable DRT concentrations and by applying the first derivative of the ratio spectra was obtained by utilizing a Δλ = 10, smoothing level (× 10), and a scaling factor of 10, then reproducible peaks were selected from the resulting derivative ratio spectra to estimate DRT. DRT amplitudes were calculated at 309.0 nm, as shown in Fig. 8.
Figure 9 illustrates the ratio spectra of variable CAFF concentrations by applying the first derivative of the ratio spectra was obtained by utilizing a Δ λ = 10, smoothing level (× 10), and a scaling factor of 10. In Fig. 10, reproducible peaks were selected from the resulting derivative ratio spectra to estimate CAFF at 285.
Figure 11 illustrates the ratio spectra of variable PAR concentrations. The first derivative spectra of the ratio spectra were obtained by a Δλ = 10, smoothing level (× 10), and a scaling factor of 10. Reproducible peaks were selected from the resulting derivative ratio spectra to determine PAR at 255.0 nm, as shown in Fig. 12.
The amplitudes of DRT, CAFF and PAR at the selected wavelengths were plotted against the corresponding drug concentrations.
Mean centering of ratio spectra spectrophotometric method (MCR) method (Method III)
After studying these parameters, it was found that the divisor had a great effect on the selectivity of determination of the studied drugs where reproducible and good results were obtained upon using concentrations of 9 μg/mL and 8 μg/mL each of CAFF and PAR (for DRT) and 8 μg/mL and 8 μg/mL each of DRT and PAR (for CAFF) and 6 μg/mL each of CAFF and DRT (for PAR) as divisors. Figures 13, 14, 15 are the calibration curves relating the mean-centered values at 309, 214, and 248 nm to the corresponding concentrations of DRT, CAFF, and PAR, respectively, have been constructed from which the regression equation parameters.
Methods optimization
-
–Effect of diluting solvent
Different diluting solvents were examined, like distilled water, methanol, ethanol, acetonitrile, and acetone, and it was found that ethanol is the best diluting solvent for the studied drugs as it gave high absorbance (Fig. 16).
-
The optimization of the derivative spectrophotometric method scanned by different values of Δλ, smoothing level and scaling factor were examined to improve resolution of this mixture. It is found that first derivative spectrophotometry with Δλ = 10, scaling factor 20 and smoothing level 10 were good parameters for DRT and CAFF resolution while for PAR resolution is second derivative scaling factor 20 smoothing level × 10.
-
The optimization of the double divisor ratio derivative come by changing the concentrations of the double divisor till reaching the best concentration for accuracy and reproducibility. The double divisor in case of DRT is 9 μg/mL CAFF and 8 μg/mL PAR, CAFF is 8 μg/mL DRT + 8 μg/mL PAR and PAR is 6 μg/mL CAFF + 6 μg/ml DRT. Changing the Δλ from 10 to 40 nm till found 10 nm is the best, smoothing level until 10 and also scaling factor was scanned like 1, 5, 10 and 20 till found that 10 is the best.
-
The optimization in the mean centering method determined through the choice of the divisor and hence the vectors that selected for mean centering and the range of the spectrum. After studying these parameters, it was found that 200–400 nm is the UV spectrophotometric range. The divisor had a great effect on the selectivity of determination of DRT, CAFF and PAR where reproducible and good results have been obtained upon using concentration of 9 μg/mL and 8 μg/mL each of CAFF and PAR (for DRT) and 8 μg/mL and 8 μg mL−1 each of DRT and PAR (for CAFF) and 6 μg/mL each of CAFF and DRT (for PAR) as divisors. DRT, CAFF and PAR could be determined at 309, 214 and 248 nm, respectively.
Method validation
The validation parameters were studied according to ICH recommendations [80].
The linearity of the proposed methods was estimated through the calibration graphs by plotting the amplitude of the first derivative versus the concentration of DRT, CAFF, and PAR at 320.0, 285, and 307 nm, respectively, in the case of the derivative method and DRT, CAFF, and PAR at 309, 288, and 255 nm respectively in case of double divisor method.
The linearity of the mean centering was estimated through the calibration graphs by plotting absorbance response versus the concentration of DRT, CAFF, and PAR at 309.0 nm, 214.0 nm, and 248 nm, respectively, in the case of the mean centering method.
The concentrations were rectilinear in the ranges of 2–20 µg/mL for DRT, 1.5–15 µg/mL for CAFF, and 2–40 µg/mL for PAR in double devisor and mean centering but PAR from 5 to 40 µg / mL in derivative method.
High correlation coefficients (r) of the regression equations, small residual standard deviation (Sy/x) and percentage relative standard deviation (%RSD) values, intercept and slope standard deviation (Sa), are all produced through statistical analysis of the data (Tables 1, 2, 3). Limits of Quantitation and detection (QL) (DL) were calculated according to ICH Q2 (R1) recommendations [80] are also abridged in Tables 1, 2, and 3.
The repeatability of the method was determined by using three concentrations (4, 8, and 10 μg/mL) for DRT and (3, 6, and 15 μg/mL) for CAFF, and (10, 20 and 40 μg/mL) for PAR 3 times intra-daily and interday using the proposed univariate methods. Good results and acceptable % RSDs (less than 2%) were obtained, as summarized in Tables 4, 5, and 6.
To evaluate the accuracy of the proposed methods for the studied drugs, whether alone or in their synthetic mixtures within their linearity ranges. The recovery percentages obtained are illustrated in Tables 7, 8, and 9 and Tables 10 and 11 for the synthetic mixtures. The suggested methods proved accurate, as revealed by the high recoveries values and low standard deviations. Statistical analysis of the results obtained by both the proposed and the comparison methods [79] was performed. F-test and t-test tested the difference between methods. The test ascertained no significant difference in accuracy and precision between the proposed and the comparison methods.
The selectivity of the method was assessed by observing any interference encountered from the tablet additives cited in the information pamphlet of the studied pharmaceutical preparation (Petro® tablets). About 0.657 g, which approximately equals the weight of one tablet of each additive including magnesium stearate, lactose monohydrate, maize starch, calcium hydrogen phosphate dihydrate, and talc was analyzed using the same procedure described for the analysis of tablets. No interference was encountered from any tablet additive, which confirms the adequate selectivity of the developed method.
Applications
Application to synthetic mixtures
The proposed methods utilized to analyze the three drugs in their 1:1.5:10 synthetic mixture in Tables 10 and 11 showed acceptable percentage recoveries for both drugs illustrated in Figs. 17 and 18 in the derivative and double divisor method, respectively.
DRT, CAFF and PAR with their synthetic mixtures in double divisor method: (A) a is 4 µg/mL DRT with b is the synthetic mixture containing 4 µg/mL DRT, (B) c is 40 µg/mL PAR with d is the synthetic mixture containing 40 µg/mL PAR, (C) e is 4.5 µg/mL CAFF with f is the synthetic mixture containing 4.5 µg/mL CAFF
Applications to pharmaceutical formulations
These spectrophotometric methods were excellent applied on the pure bulk powder and on the pharmaceutical preparation: Petro®, which contains 40 mg DRT, 60 mg of CAFF, and 400 mg PAR with good accuracy and precision. There was no significant difference after comparing the proposed method with the published method [79] after calculating the student’s t-test and F-value [81] provided in Tables 12, 13, and 14.
Greenness assessment
Due to the considerable usage of organic solvents in analytical processes, going green can be very difficult. The greenness of these methods was assessed in three different ways.Firstly, Green analytical procedure index (GAPI) [82] The green profiles for the proposed spectrofluorometric methods using the GAPI tool are presented in (Table 15). The 5th parameter was shaded yellow as there was a bit of sample preparation as filtration. Field No. 15 in all techniques had red coloring because there was no waste treatment and the amount of waste was between 1 and 10 mL, thus it was tinted yellow.
Analytical eco scale is another quantitative assessment tool Van-Aken et al. [83] published. The proposed methods scored 93, as shown in Table 15. This method is excellent regarding the analytical eco-scale criteria. The penalty points were calculated by the national fire protection association (NFPA) [84].
The National Environmental Method Index (NEMI), an outdated qualitative tool, is the final one [85]. It describes the greenness through a pictogram divided into four quadrants (Table 15). All four quadrants are green colored as no reagents or chemicals are used except ethanol, an eco-friendly solvent. The created approach works well with the three green analytical chemistry tools, which explains why these procedures are environmentally benign, straightforward, quick, and sensitive.
In-vitro dissolution test
Dissolution testing has become an essential tool in the pharmaceutical industry at various stages of development, manufacturing and marketing. The in-vitro dissolution profile of Petro® tablets was performed using paddle method. The dissolution media are: HCl solution of pH 1.2, acetate buffer of pH 4.5, phosphate buffer of 6.8 and water [8]. The tablets were placed in 800 ml of medium at 37 °C with stirring speed of 75 rpm. Then, 1 ml of samples were withdrawn out at five, ten, twenty, thirty, sixty and ninety min, and same volume of medium was supplemented to maintain constant medium volume. After filtration using 0.22 μm syringe filters, the samples were analyzed adopting the proposed derivative method according to the procedure 2.4.2. Calibration graphs development. It was found that the release of DRT, CAFF and PAR from its tablets depended on the pH Fig. 19.
Conclusion
A rapid and simple spectrophotometric approach was devised to simultaneously determine DRT, CAFF, and PAR in response to the demands of quality control laboratories. This straightforward, inexpensive method may be preferable to more expensive, sophisticated ones for routine examination of the examined medications in the co-formulated dosage form.
Availability of data and materials
All data generated or analysed during this study are included in this published article.
References
Lin SY. Ultraviolet spectrophotometry. In: Lin Stephen Y, Dence Carlton W, editors. Methods in lignin chemistry. Berlin: Springer Berlin Heidelberg; 1992.
El-Malla Kamal S, Hammad SHA. A review on UV spectrophotometric methods for simultaneous multicomponent analysis. Eur J Pharma Med Res. 2016;3:348–60.
Bosch O, Sanchez CRF. Recent developments in derivative ultraviolet/visible absorption spectrophotometry. Anal Chim Acta. 2004;518(1):1–24.
Dinç E, Onur F. Application of a new spectrophotometric method for the analysis of a ternary mixture containing metamizol, paracetamol and caffeine in tablets. Anal Chim Acta. 1998;359(1–2):93–106.
Afkhami A, Bahram M. Mean centering of ratio spectra as a new spectrophotometric method for the analysis of binary and ternary mixtures. Talanta. 2005;66(3):712–20.
O’Neil JM. The merck index. 14th ed. Rahway: Merck and Co Inc.; 2006.
British Pharmacopoeia. 2016. London: The stationary office.
The United States Pharmacopeia. US Pharmacopeial Convention. Rockville: The National Formulary 27; 2009.
Martindale SS. The complete drug reference. 37th edn ed. Vol. (7). London, UK: The Pharmaceutical Press; 11126.
Abdellatef HE, Ayad MM, Soliman MS, Youssef NF. Spectrophotometric and spectrodensitometric determination of paracetamol and drotaverine HCl in combination. J Spectrochim Acta Part A: Mol Biomol Spectrosc. 2007;66(4–5):1147–51.
Metwally FH. Simultaneous determination of nifuroxazide and drotaverine hydrochloride in pharmaceutical preparations by bivariate and multivariate spectral analysis. J Spectrochim Acta Part A: Mol Biomol Spectrosc. 2008;69(2):343–9.
Daabees H. Selective differential spectrophotometric methods for determination of niclosamide and drotaverine hydrochloride. Anal Lett. 2000. https://doi.org/10.1080/00032710008543080.
Dahivelkar P, Mahajan VK, Bari SB, Shirkhedkar AA, Fursule RA, Surana SJ. Simultaneous derivative and multicomponent spectrophotometric determination of drotaverine hydrochloride and mefenamic acid in tablets. J Indian J Pharm Sci. 2007;69(6):812.
El-Wasseef DR, El-Sherbiny D, Eid M, Belal F. Spectrofluorometric determination of drotaverine hydrochloride in pharmaceutical preparations. J Anal Lett. 2008;41(13):2354–62.
El-Saharty Y, Metwaly FH, Refaat M, El-Khateeb SZ. Application of new membrane selective electrodes for the determination of drotaverine hydrochloride in tablets and plasma. J Pharm Biomed Anal. 2006;41(3):720–4.
Jain R, Jadon N, Rather JA. Voltammetric behaviour of drotaverine hydrochloride in surfactant media and its enhancement determination in Tween-20. J Colloids Surfaces B: Biointerfaces. 2011;82(2):333–9.
Zayed S, Issa YM. Cathodic adsorptive stripping voltammetry of drotaverine hydrochloride and its determination in tablets and human urine by differential pulse voltammetry. J Bioelectrochem. 2009;75(1):9–12.
Jain R, Jadon N. Voltammetric quantification of antispasmodic drug drotaverine hydrochloride in human serum. J Curr Pharm Anal. 2015;11(2):145–54.
Rele RV. A non-aqueous potentiometric titration method for validation of drotaverine hydrochloride from pharmaceutical dosages. J Int J Chem Res. 2018. https://doi.org/10.2090/IJCTR.2018.111123.
Issa YM, Hassouna MEM, Zayed AG. Simultaneous determination of paracetamol, caffeine, domperidone, ergotamine tartrate, propyphenazone, and drotaverine HCl by high performance liquid chromatography. J Liq Chromatogr Relat Technol. 2012;35(15):2148–61.
Wahab S, Mohamed FP, Mohamed HS, Chandran M, Kadher MS. Development of RP-HPLC method for the simultaneous determination of mefenamic acid and drotaverine HCl combined tablet dosage form. J Int J Pharm Sci. 2011;3(1):115–7.
Dahivelkar PP, Bhoir SI, Bari SB, Surana SJ, Bhagwat AM. Simultaneous determination of diclofenac potassium and drotaverine hydrochloride in human plasma using reversed-phase high-performance liquid chromatography. J Chromatogr Sci. 2012;50(8):694–701.
Maher HM, Belal TS. HPLC-DAD stability indicating determination of the fixed-dose combination of nifuroxazide and drotaverine hydrochloride in capsules. J Liq Chromatogr Relat Technol. 2012;35(14):2001–20.
Azhlwar S, Ravi TK. Stability indicating HPLC method for simultaneous determination of drotaverine and aceclofenac. J Int J Pharm Pharm Sc. 2011;3(1):245–50.
Sharma S, Sharma MC. Development and validation of new analytical methods for simultaneous estimation of drotaverine hydrochloride in combination with omeprazole in a pharmaceutical dosage form. J Arab J Chem. 2017;10:S397–403.
Snigdha D. Development and validation of stability indicating RP-HPLC method for the determination of drotaverine hydrochloride. J International Journal of Pharmaceutics Drug Analysis, 2015: p. 234–245.
Vancea S, Gáll Z, Donáth-N G, Borka- R. Rapid LC–MS/MS method for determination of drotaverine in a bioequivalence study. J Pharm Biomed Anal. 2014;98:417–23.
Pinnamaneni P, Elphine P, Suresh PVA, Nagamalleswari G, Nadendla RR. Method development and validation for simultaneous estimation of drotaverine hydrochloride and aceclofenac in bulk and formulation by RP-HPLC. J World J Pharma Pharm Sci. 2015;4(12):1351–8.
Kumar RV, Rao VU, Kumar N, Subbaiah BV. A novel, rapid, and validated stability-indicating UPLC method for the estimation of drotaverine hydrochloride and ibuprofen impurities in oral solid dosage form. J Sci Pharm. 2015;83(4):567–81.
Rani GT, Sankar DG, Kadgapathi P, Satyanarayana B. Development and validation of RP-HPLC method for estimation of drotaverine hydrochloride and nimesulide in pharmaceutical dosage form. J Asian J Res Chem. 2011;4(1):151–5.
Balan P, Nimila IC, Chundi S, Movva VR, Rajasekar SA. Validated RP-HPLC method for estimation of drotaverine HCL in pharmaceutical tablet dosage form. J Int J Pharm Pharm Sci. 2012;3:77–80.
Abdellatef HE, Ayad MM, Soliman SM, Youssef NF. Spectrophotometric and spectrodensitometric determination of paracetamol and drotaverine HCl in combination. J Spectrochim Acta Part A: Mol Biomol Spectrosc. 2007;66(4–5):1147–51.
Metwally FH, El-Saharty YS, Refaat M, El-Khateeb SZ. Application of derivative, derivative ratio, and multivariate spectral analysis and thin-layer chomatography-densitometry for determination of a ternary mixture containing drotaverine hydrochloride, caffeine, and paracetamol. J AOAC Int. 2007;90(2):391–404.
George AJ. Central nervous system stimulants. Best Pract Res Clin Endocrinol Metab. 2000;14(1):79–88.
Alpdoğan G, Karabina K, Sungur S. Derivative spectrophotometric determination of caffeine in some beverages. J Turkish J Chem. 2002;26(2):295–302.
Dinç E, Baleanu D, Onur F. Spectrophotometric multicomponent analysis of a mixture of metamizol, acetaminophen and caffeine in pharmaceutical formulations by two chemometric techniques. J Pharm Biomed Anal. 2001;26(5):949–57.
Singh DK, Sahu A. Spectrophotometric determination of caffeine and theophylline in pure alkaloids and its application in pharmaceutical formulations. Anal Biochem. 2006;349(2):176–80.
Khoshayand MR, Abdollahi H, Shariatpanahi M, Saadatfard A, Mohammadi A. Simultaneous spectrophotometric determination of paracetamol, ibuprofen and caffeine in pharmaceuticals by chemometric methods. Spectrochim Acta Part A Mol Biomol Spectrosc. 2008;70(3):491–9.
Aktaş AH, Kitiş F. Spectrophotometric simultaneous determination of caffeine and paracetamol in commercial pharmaceutical by principal component regression, partial least squares and artificial neural networks chemometric methods. Croat Chem Acta. 2014;87:69–74.
Moreira AB, Dias ILT, Neto GO, Zagatto EAG, Kubota LT. Simultaneous spectrofluorimetric determination of paracetamol and caffeine in pharmaceutical preparations in solid-phase using partial least squares multivariate calibration. Anal Lett. 2006;39(2):349–60.
Alvi SN, Hammami MM. Validated HPLC method for determination of caffeine level in human plasma using synthetic plasma: application to bioavailability studies. J Chromatogr Sci. 2011;49(4):292–6.
Franeta J, et al. HPLC assay of acetylsalicylic acid, paracetamol, caffeine and phenobarbital in tablets. Il Farmaco. 2002;57(9):709–13.
Altun ML. HPLC method for the analysis of paracetamol, caffeine and dipyrone. Turk J Chem. 2002;26(4):521–8.
Ali MM, et al. Determination of caffeine in some sudanese beverages by high performance liquid chromatography. Pak J Nutr. 2012;11(4):336.
Cunha RR, et al. Simultaneous determination of caffeine, paracetamol, and ibuprofen in pharmaceutical formulations by high-performance liquid chromatography with UV detection and by capillary electrophoresis with conductivity detection. J Sep Sci. 2015;38(10):1657–62.
Leacock RE, Stankus JJ, Davis JM. Simultaneous determination of caffeine and vitamin B6 in energy drinks by high-performance liquid chromatography (HPLC). J Chem Educ. 2011;88(2):232–4.
Dewani A, et al. Development and validation of a novel RP-HPLC method for simultaneous determination of paracetamol, phenylephrine hydrochloride, caffeine, cetirizine and nimesulide in tablet formulation. Arab J Chem. 2015;8(4):591–8.
Belal TS, et al. High performance liquid chromatographic determination of the ternary mixture of caffeine, dipyrone and drotaverine hydrochloride in tablet dosage form. J Appl Pharm Sci. 2014;4(6):33.
Rodrigues CI, et al. Application of solid-phase extraction to brewed coffee caffeine and organic acid determination by UV/HPLC. J Food Compos Anal. 2007;20(5):440–8.
Fernandez P, et al. HPLC determination of catechins and caffeine in tea. Differentiation of green, black and instant teas. Analyst. 2000;125(3):421–5.
Wanyika H, et al. Determination of caffeine content of tea and instant coffee brands found in the Kenyan market. Afr J Food Sci. 2010;4(6):353–8.
Shishov A, et al. An automated homogeneous liquid-liquid microextraction based on deep eutectic solvent for the HPLC-UV determination of caffeine in beverages. Microchem J. 2019;144:469–73.
Amiri-A M, Raoof JB, Ojani R. A highly sensitive electrochemical sensor for simultaneous voltammetric determination of noradrenaline, acetaminophen, xanthine and caffeine based on a flavonoid nanostructured modified glassy carbon electrode. Sens Actuators B Chem. 2014;192:634–41.
Sun J-Y, Huang K-J, Wei S-Y, Wu Z-W, Ren F-P. A graphene-based electrochemical sensor for sensitive determination of caffeine. Colloids Surfaces B Biointerfaces. 2011;84(2):421–6.
Monteiro MKS, Da Silva DR, Quiroz MA, Vilar VJP, Martínez-H CA, Dos Santos EV. Applicability of cork as novel modifiers to develop electrochemical sensor for caffeine determination. Materials. 2021;14(1):37.
Švorc L, Tomčík P, Svítková J, Rievaj M, Bustin D. Voltammetric determination of caffeine in beverage samples on bare boron-doped diamond electrode. Food Chem. 2012;135(3):1198–204.
Shrestha BR, Pradhananga RR. Spectrophotometric method for the determination of paracetamol. J Nepal Chem Soc. 2009;24:39–44.
Ghulam M, Izhar H, Shujaat AK, Arham S, Arshad M, Muhammad HHB, Kalsoom F, Malik NS. Development of a UV-spectrophotometric method for the simultaneous determination of aspirin and paracetamol in tablets. J Sci Res Essays. 2011;6(2):417–21.
Rodenas V, Garcıa MS, Sanchez-P C, Albero MI. Simultaneous determination of propacetamol and paracetamol by derivative spectrophotometry. J Talanta. 2000;52(3):517–23.
Sena MM, Poppi RJ. N-way PLS applied to simultaneous spectrophotometric determination of acetylsalicylic acid, paracetamol and caffeine. J Pharm Biomed Anal. 2004;34(1):27–34.
Vichare V, Mujgond P, Tambe V, Dhole SN. Simultaneous spectrophotometric determination of paracetamol and caffeine in tablet formulation. J Int J PharmTech Res. 2010;2(4):2512–6.
Moreira AB, Oliveira HPM, Atvars TDZ, Dias ILT, Neto Graciliano O, Zagatto EAG, Kubota LT. Direct determination of paracetamol in powdered pharmaceutical samples by fluorescence spectroscopy. J Anal Chim Acta. 2005;539(1–2):257–61.
Goyal RN, Gupta VK, Oyama M, Bachheti N. Differential pulse voltammetric determination of paracetamol at nanogold modified indium tin oxide electrode. J Electrochem Commun. 2005;7(8):803–7.
Goyal RN, Gupta VK, Chatterjee S. Voltammetric biosensors for the determination of paracetamol at carbon nanotube modified pyrolytic graphite electrode. J Sens Actuators B Chem. 2010;149(1):252–8.
Goyal RN, Singh SP. Voltammetric determination of paracetamol at C60-modified glassy carbon electrode. J Electrochim Acta. 2006;51(15):3008–12.
Bayram E, Akyilmaz E. Development of a new microbial biosensor based on conductive polymer/multiwalled carbon nanotube and its application to paracetamol determination. J Sens Actuators B Chem. 2016;233:409–18.
Özcan L, Şahin Y. Determination of paracetamol based on electropolymerized-molecularly imprinted polypyrrole modified pencil graphite electrode. J Sens Actuators B Chem. 2007;127(2):362–9.
Lourenção BC, Medeiros RA, Rocha-Filho RC, Mazo LH, Fatibello-Filho O. Simultaneous voltammetric determination of paracetamol and caffeine in pharmaceutical formulations using a boron-doped diamond electrode. Talanta. 2009;78(3):748–52.
Avinash B, Ravikumar CR, Kumar MRA, Nagaswarupa HP, Santosh MS, Bhatt A, Kuznetsov D. Nano CuO: electrochemical sensor for the determination of paracetamol and d-glucose. J Phys Chem Solids. 2019. https://doi.org/10.1016/j.jpcs.2019.06.012.
Belal T, Awad T, Clark CR. Determination of paracetamol and tramadol hydrochloride in pharmaceutical mixture using HPLC and GC-MS. J Chromatogr Sci. 2009;47(10):849–54.
Monser L, Darghouth F. Simultaneous LC determination of paracetamol and related compounds in pharmaceutical formulations using a carbon-based column. J Pharm Biomed Anal. 2002;27:851–60.
Erk N, Ozkan Y, Banoğlu E, Ozkan SA, Sentürk Z. Simultaneous determination of paracetamol and methocarbamol in tablets by ratio spectra derivative spectrophotometry and LC. J Pharm Biomed Anal. 2001;24(3):469–75.
Shaikh KA, Devkhile AB. Simultaneous determination of aceclofenac, paracetamol, and chlorzoxazone by RP-HPLC in pharmaceutical dosage form. J Chromatogr Sci. 2008;46(7):649–52.
Abdelaleem EA, Abdelwahab NS. Validated stability indicating RP-HPLC method for determination of paracetamol, methocarbamol and their related substances. Anal Methods. 2013;5(2):541–5.
Ruengsitagoon W, Liawruangrath S, Townshend A. Flow injection chemiluminescence determination of paracetamol. Talanta. 2006;69(4):976–83.
Llorent-M E, Satinsky D, Solich P, Ortega-B P, Molina-D A. Fluorimetric SIA optosensing in pharmaceutical analysis: determination of paracetamol. J Pharm Biomed Anal. 2007;45:318–21.
Capella-P M-E, Bose D, Rubert MF, Esteve-R J. Optimization of a capillary zone electrophoresis method by using a central composite factorial design for the determination of codeine and paracetamol in pharmaceuticals. J Chromatogr. 2006;839(1–2):95–101.
Belal F, El-Din Mohie S, Tolba M, El-Awady M, Elmansi H. Analysis of four antimigraine drugs in two ternary mixtures by sweeping-micellar electrokinetic chromatography with retention factor gradient effect and dynamic pH junction. Microchem J. 2016;127:11–21.
Belal F, Sharaf El-Din M, Tolba M, Elmansi H. Determination of two ternary mixtures for migraine treatment using HPLC method with ultra violet detection. J Sep Sci Technol. 2015;50(4):592–603.
ICH Harmonized Tripartite Guideline,Validation of Analytical Procedures: Text and Methodology, Q2(R1), Current Step 4 Version,Parent Guidelines on Methodology. www.ich.org/page/quality-guidelines. Acessed 01–4–2021 Dated November 6 1996, Incorporated in November 2005.
Miller JC, Miller JN. 5th edition, Pearson Education Limited, Harlow, England, Statistics and Chemometrics for Analytical Chemistry; 5th edn. Harlow, UK: Pearson Education Limited 2005.
Płotka-W J. A new tool for the evaluation of the analytical procedure: green analytical procedure index. J Talanta. 2018. https://doi.org/10.1016/j.talanta.2018.01.013.
Koen A V, Lucjan S, Luc P. EcoScale, a semi-quantitative tool to select an organic preparation based on economical and ecological parameters. J Beilstein Journal of Organic Chemistry. 2006.
NFPA1852, standard on selection, Care, and Maintanence of open-circuit self contained Breathing Apparatus (SCBA). 2013
Marek T. Metrics for green analytical chemistry. J Anal Methods. 2016. https://doi.org/10.1039/C6AY00478D.
Acknowledgements
Not applicable.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
R.G carried out the laboratory work, participatd in data analysis and participated in the design of the study; M. I. E and M. M. T drafted the manuscript, carried out the statistical analysis, conceived of the study and followed up the experimental work; F. I and M. I. E coordinated the study, participated in data analysis and supervised the whole work. All authors gave final approval for publication.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Ghonim, R., Tolba, M.M., Ibrahim, F. et al. Smart green spectrophotometric assay of the ternary mixture of drotaverine, caffeine and paracetamol in their pharmaceutical dosage form. BMC Chemistry 17, 181 (2023). https://doi.org/10.1186/s13065-023-01097-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13065-023-01097-9