Abstract
Background
Chlorthalidone is a diuretic medicinal agent used to treat hypertension. Lowering hypertension results in the prevention of strokes, heart attacks and kidney-related problems.
Results
In this work, the spectrophotometric investigation was employed to examine the reaction between chlorthalidone and 1, 2-naphthoquinone-4-sulfonate. A reaction is performed at the alkaline environment (pH 9.2) and heated at a moderate temperature of 60 ± 1 °C using a water bath. The red colour product was produced, and it displayed a maximum absorbance at 440.50 nm. Further, the advancement of response conditions was explored by UV/visible spectrophotometer. The stoichiometric of the reaction was considered by using Job’s plot and the reaction mechanism of the proposed work was drawn. The established approach obeyed the calibration curve in the concentration range of 2–12 μg/mL with a regression coefficient superior to 0.994. The accuracy of the method, assessed by employing the % recovery, was in the range of 99.06–99.60% with the % relative standard deviation being less than 2%. The limit of detection and limit of quantification were 0.58 μg/mL and 1.72 μg/mL, respectively.
Conclusion
A UV/visible spectrophotometric investigation for the analysis of the chlorthalidone in the tablet matrix was successfully established and validated. The established investigation was found to be simple and accurate, and because of its ease of use, the proposed strategy could be applied for quality control investigation of chlorthalidone in pharmaceutical matrices.
Similar content being viewed by others
Background
Chlorthalidone (CHL), chemically 2-chloro-5-(1-hydroxy-3-oxo-2H-isoindol-1-yl) benzenesulfonamide;N-(2,6-dichlorophenyl)-4,5-dihydro-1H-imidazol-2-amine (Fig. 1), is used in the treatment of hypertension [1]. CHL is a phthalimidine diuretic that shows an extended duration of action up to 72 h after an administration of single-dose, and it shows the antidiuretic effect by inhibiting Na+ and Cl- transporters in distal convoluted tubules and increasing Na+ and Cl- excretion [2, 3]. Literature survey demonstrated that several analytical methods have been established to investigate CHL in pure form and pharmaceutical preparations alone or combined with other medicinal agents. These reported methods include high-performance liquid chromatography [4,5,6,7,8,9,10,11,12,13,14,15], high-performance thin-layer chromatography [16,17,18], spectrophotometry and spectrofluorometry [19,20,21,22,23,24,25,26,27,28,29,30,31,32,33], capillary electrophoresis [34,35,36], and potentiometry [37]. Nowadays, 1, 2-naphthoquinone-4-sulfonate is a choice of derivatizing reagent employed for the estimation of drugs containing primary and secondary amino groups using spectrophotometry and spectrofluorometry. These types of drugs are easily reacting with NQS under the alkaline medium and at moderate temperatures to yield a derivative product which can be efficiently detected through spectrofluorometric and spectrophotometric methods [38]. So far in the literature, no research work was accounted for being related to the utilization of UV/vis-spectrophotometric technique for assessment of CHL in tablet dosage form using 1, 2-naphthoquinone-4-sulfonate. Although several UV/vis-spectrophotometric methods have been reported for estimation of CHL, to our knowledge, in all approaches, different wavelengths were used for the analysis of CHL. Therefore, there is a vital need for the development of a specific UV/vis-spectrophotometric method which uses a fixed wavelength for analysis of CHL. Till date, there is no single report available on literature for the analysis of CHL using NQS. Hence, it was decided to explore 1, 2-naphthoquinone-4-sulfonate derivatizing agent for the establishment of sensitive and precise UV/vis-spectrophotometric investigation for CHL analysis in the tablet matrix. Moreover, the stoichiometric ratio of reaction between CHL and 1, 2-naphthoquinone-4-sulfonate was successfully established using Job’s method.
Methods
Chemicals
Chemicals and materials used in proposed work include working standard CHL which was obtained as a gift sample from the J. B. Chemicals and Pharmaceuticals Ltd. Ankleshwar, India. 1, 2-Naphthoquinone-4-sulfonate sodium salt and HPLC grade methanol were purchased from Sigma-Aldrich Mumbai, India.
Instrumentation
A spectrophotometric optimization was performed on a double-beam UV/visible spectrophotometer (Shimadzu, Japan), with a deuterium lamp, electronic balance (Model Shimadzu AUX 120), pH meter (model EQ 621, Equiptronics, India), and thermostatically monitored water bath.
Preparation of stock standard solution
The stock standard solution was prepared by accurately dissolving 10 mg of CHL in 10 mL of methanol to the obtained concentration of 1 mg/mL. From it, appropriate volume was further diluted with the same solvent to achieve a concentration of 0.1 mg/mL.
Preparation of reagent
1, 2-Naphthoquinone-4-sulfonate (0.5% w/v)
An accurate weight of 50 mg of 1, 2-naphthoquinone-4-sulfonate was transferred into a 10-mL amber colour volumetric flask, dissolved in 5 mL of water, and sonicated for 10 min, and further volume was adjusted to mark with the same solvent to an obtained concentration of 0.5% w/v.
Preparation of buffer solution (pH 9.2)
The buffer solution of pH 9.2 was prepared by dissolving 7.645 g of sodium bicarbonate and 0.954 g of sodium carbonate in 800 mL of water, and finally, the volume was adjusted up to 1000 mL using the water [39].
The general protocol for optimization of chlorthalidone-1, 2-naphthoquinone-4-sulfonate complex
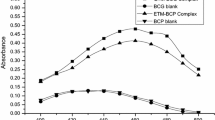
A 1 mL of a solution of CHL was transferred into 10 mL of the calibrated flask; subsequently, 1 mL of sodium carbonate buffer (pH 9.2) and 1 mL of 0.5% 1, 2-naphthoquinone-4-sulfonate solution were added into the calibrated flask. The resulting solution was kept on a water bath at 60 ± 1 °C for 20 min. After heating, the reaction solution was cooled at room temperature and the volume of the calibrated flask was adjusted up to the mark up with methanol. The CHL-1, 2-naphthoquinone-4-sulfonate complex depicted maximum absorbance at a wavelength of 440.50 nm with methanol as the blank solvent. The UV/visible spectrum of CHL-1, 2-naphthoquinone-4-sulfonate complex was shown in Fig. 2.
Measurement of the stoichiometric ratio of reaction between chlorthalidone-1, 2-naphthoquinone-4-sulfonate
Job’s method for continuous variation was successfully applied to determine the stoichiometric of a reaction within CHL-1, 2-naphthoquinone-4-sulfonate under operative condition [40, 41]. An equimolar methanolic solution of CHL and aqueous solutions of 1, 2-naphthoquinone-4-sulfonate of 0.002 M (2 × 10−3 M) were prepared separately in the calibrated flask. Soon after, a set of 10 mL of portions of the equimolar solutions of CHL and 1, 2-naphthoquinone-4-sulfonate were mixed in calibrated flasks in different proportions (9:1, 8:.2, 7:3….1:9, 2:8, 3:7) containing 1 mL of buffer solution of (pH 9.2). The subsequent arrangements were additionally drawn closer for a general convention for estimation of the stoichiometric proportion of response.
Results
Quantification of wavelength
Initially, the UV/vis absorption spectrum of CHL was measured in methanol as a reference solvent using a concentration of a 10 μg/mL. From there, it was observed that the CHL show maximum absorbance (0.9847) at 214.80 nm. The determination of CHL in tablets was quite difficult using this wavelength (214.80 nm) because of potential interference from common excipients. Hence, an attempt to shift the lambda max of CHL at higher wavelength was needed for its determination. Therefore, we carried out the reaction of CHL with 1, 2-naphthoquinone-4-sulfonate using an alkaline medium at a temperature of 60 ± 1 °C for 20 min. After the reaction, red-orange colour solution (product) was observed and the UV/vis spectrum of the product was determined using methanol as a blank. It was observed that the lambda max of CHL has shifted to longer wavelength, i.e. 440.50 nm. Similarly, a blank determination was performed to study the effect of reagent; from these, it was noticed that the blank solution shows lambda max at 360 nm for 1, 2-naphthoquinone-4-sulphonic acid. To estimate the CHL in tablets and to avoid interference from excipients, 440.50 nm was selected as the wavelength for rest of the investigation. The stoichiometric reaction between drug and reagent was examined by Job’s method, under the established optimized conditions. The symmetrical bell-shaped curve (Job plot) was plotted (Fig. 3), and from these, it is clear that the stoichiometric ratio is 1:1.
Optimization of reaction condition for chlorthalidone-1, 2-naphthoquinone-4-sulfonate complex
Effect of temperature
The impact of temperature on the reaction among CHL and 1, 2-naphthoquinone-4-sulfonate was estimated by performing the reaction at distinct temperatures (40–80 °C). The absorbance of reaction increased with the increase in temperature up to a certain level, and then the absorbance declined. The decrease in the absorbance of a complex was due to the instability of the reaction product at a higher temperature (85–95 °C). The higher absorbance of a reaction product was achieved at 60 ± 1 °C, and absorbance was remained steady up to 80 °C.
Effect of time
The impact of time on the formation of the complex was examined by permitting the reaction for a changing time. The most favourable heating time for the development of complex was 20 min as depicted in Fig. 4.
Effect of pH
The effect of pH on the development of complex was investigated in different pH ranges (9.2–10.6) with carbonate-bicarbonate buffer. The pH of the reaction is the most stringent factor because the absorbance intensity of the complex was directly depending on the pH of the reaction medium. The maximum absorption of the complex was achieved using the pH 9.2, and the absorption of the complex was remained steady for the pH 10.6. Therefore, the pH 9.2 buffer solution was used for the rest of the analysis as illustrated in Fig. 5.
Effect of 1, 2-naphthoquinone-4-sulfonate concentration (% w/v)
While measuring the impact of concentration of 1, 2-naphthoquinone-4-sulfonate on the complex formation reaction, it was noticed that the complex of reaction depended on the concentration of 1, 2-naphthoquinone-4-sulphonic acid. The concentration of 1, 2-naphthoquinone-4-sulfonate increased as the absorbance intensity of the complex formation reaction was also increased. Excellent results were obtained using 0.5% w/v concentration of 1, 2-naphthoquinone-4-sulphonic acid. Therefore, the 0.5% w/v concentration of 1, 2-naphthoquinone-4-sulfonate was selected for the rest of the analysis as shown in Fig. 6.
Stability of complex
The stability of the reaction product was checked by measuring the absorbance intensity of the complex at a different time at room temperature. From there, it was revealed that the complex was stable for 1 h at room temperature.
Influence of diluting solvent
Influence of diluting solvent was studied using water and methanol. In diluting the reaction solution with water, the colour intensity of the reaction solution was changed because of the instability of the reaction product in water. Diluting the reaction solution with methanol gave the maximum intensity of the reaction product, and hence, the methanol was used as the diluting solvent for the rest of the analysis. The reaction scheme for CHL with 1, 2-naphthoquinone-4-sulfonate was depicted in Fig. 7.
Validation of the commenced method
The established method was verified for linearity, accuracy, precision, sensitivity, and robustness as per the recommendations of an International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use (ICH) guideline [42].
Linearity study
To establish the calibration curve of the method, accurate volumes in the range of 2.0–12 mL were transferred from a secondary stock solution into a series of 10-mL calibrated flask and performed as per general protocol to obtain concentrations in the range of 2–12 μg/mL. All measurements were repeated five times for each concentration, and calibration plot was constructed by the plotting concentration of CHL-1, 2-naphthoquinone-4-sulfonate (μg/mL) complex against absorbance. A linear regression equation y = 8.77x + 7.51 was found after plotting calibration curve with coefficient correlation (r2 = 0.994).
Accuracy
The accuracy of the proposed analysis was executed using the standard addition method, where a known amount of the drug standard was employed in three distinct levels 80, 100, and 120% to tablet matrix. Accuracy of this study was measured in terms of % recovery.
Precision
The precision of the established method is demonstrated in terms of intra-day precision and inter-day precision. The repeatability of the established method for CHL-1, 2-naphthoquinone-4-sulfonate complex was assessed by analyzing the concentration of 10 μg/mL, and (%) RSD of absorption spectra was calculated for CHL-1, 2-naphthoquinone-4-sulfonate complex. Intra-day variation was measured by three replicates of three different concentrations of 4, 5, and 6 μg/mL which were analyzed on the same day and three successive days for inter-day precision.
Sensitivity
The sensitivity of the developed method was determined by calculating the limit of detection (LOD) and limit of quantification (LOQ). For determination of LOD and LOQ, different concentrations in the range of 2–4.5 μg/mL were prepared and investigated by using the general protocol. The limit of detection and limit of quantification were estimated using the formula LOD = 3.3 (SD)/S and LOQ = 10 (SD)/S, where SD = standard deviation of response and S = the slope of the calibration plot. The LOD and LOQ of the proposed method were found to be 0.58 μg/mL and 1.72 μg/mL for CHL-1, 2-naphthoquinone-4-sulfonate complex.
Robustness
Robustness of the method was studied by determining the impact of small but deliberate variation in independent variables and investigating its effect on the performance of the method. For determination of the robustness of the method, three different independent variables were selected as the volume of 1, 2-naphthoquinone-4-sulfonate solution (0.8–1.2 mL), pH of buffer solution (9.0–9.4), and water bath temperature (58–62 °C). The results of the robustness study were shown in Table 1, and a robustness study was performed using a sample solution of 10 μg/mL.
Application of the proposed method for analysis of chlorthalidone in a pharmaceutical preparation
For the preparation of the sample solution, ten tablets were taken (Thalidon-6.25 tablets, 6.25 mg/tablet), weighed, and finally powdered with mortar and pestle. An accurate weight powder drug equivalent to one tablet (6.25 mg) was taken into a 100-mL volumetric flask, dissolved in 50 mL of methanol and sonicated for 10 min. Further, the volume of the flask was made up to mark with the same solvent. The resulting solution was filtered through a 0.45-μm filter (Millifilter, Milford, MA, USA). An accurate volume was further diluted with the methanol to obtain a concentration of 6 μg/mL. A sample solution of 6 μg/mL was prepared and subjected to estimation of CHL in the tablet matrix. The result of the determination of CHL in a pharmaceutical formulation was shown in Table 2.
Discussion
CHL is effectively being used to treat hypertension and fluid retention developed due to different conditions like heart diseases. In commenced research work, a complex of 1, 2-naphthoquinone-4-sulfonate and CHL was effectively established. A red colour product was obtained in an alkaline environment (pH 9.2) and at a moderate temperature of 60 ± 1 °C for 20 min which obeyed maximum absorbance at wavelength 440.50 nm. After establishing the complex, the reaction conditions for CHL-1, 2-naphthoquinone-4-sulfonate complex were optimized for effect of temperature, effect of time, effect of pH, effect of 1, 2-naphthoquinone-4-sulfonate concentration (% w/v), the stability of complex, and influence of diluting solvent. Then, the stoichiometric reaction between drug and reagent was studied by using Job’s method under investigated conditions. After that, the symmetrical bell-shaped curve (Job plot) was constructed, and from these, it is concluded that the stoichiometric ratio is 1:1 [43].
CHL-1, 2-naphthoquinone-4-sulfonate complex obeyed good determination coefficient more than (r2=0.99) in the given concentration range of 2–12 μg/mL. Accuracy was appraised as a % recovery study at three distinct levels, i.e. 80, 100, and 120%. % Recovery study was performed using standard addition method demonstrating excellent % recovery at three distinct levels in the range of 99.06–99.60%, and their percentage RSD was less than 2 satisfying the acceptance criteria for % recovery study. The precision of the present investigation was evaluated for intra-day, inter-day, and repeatability and was acknowledge as RSD (%). The intra-day and inter-day precision were studied using the 4, 5, and 6 μg/mL concentrations, and repeatability was at 10 μg/mL and demonstrated RSD (%) less than 2% suggesting precision of the investigation. LOD and LOQ were found to be 0.58 μg and 1.72 μg, respectively. The small values of LOD and LOQ show the adequate sensitivity of the investigation. Robustness experiment for the proposed investigation has appraised the impact of independent variables on the CHL-1, 2-naphthoquinone-4-sulfonate complex. The independent variables selected for this experiment are volume of 1, 2-naphthoquinone-4-sulfonate solution, pH of buffer solution, and water bath temperature. From this, it was observed that a small variation in the independent variables did not show any impact on response indicating the robustness of investigation [44].
The tablet matrix (Thalidon-6.25 tablets) containing CHL (6.25 mg/tablet) when studying using the proposed investigation displayed excellent recovery. The amount of CHL in the tablet matrix was found to be 99.17 ± 0.40%. Form this, it was observed that none of the matrix ingredients interferes with CHL-1, 2-naphthoquinone-4-sulfonate complex. Therefore, the proposed method could be applied for quality control analysis of CHL in a pharmaceutical formulation.
The summary of validation parameters for design investigation is summarized in Table 3.
Conclusion
A simple, specific, and accurate spectrophotometric method has been developed and validated for the determination of CHL in tablet matrix. In the present study, 1, 2-naphthoquinone-4-sulfonate was successfully employed for the determination of CHL and the proposed method is the first method for determination of CHL in tablet matrix using 1, 2-naphthoquinone-4-sulphonic acid; the established method was efficiently applied for the determination of CHL in the tablet matrix without hindrance from excipients.
Availability of data and materials
All data and materials are available upon request
Abbreviations
- CHL:
-
Chlorthalidone
LOD
Limit of detection and limit
LOQ
Limit of quantitation
References
Davies DL, Wilson GM (1975) Diuretics: mechanism of action and clinical application. Drugs 9:178–226
Mees ED, Geyskes GG (1964) A comparative study of the diuretics chlorthalidonum and cyclopenthiazidum. Acta Medica Scandinavica 175:703–713
Roush GC, Buddharaju V, Ernst ME, Holford TR (2013) Chlorthalidone: mechanisms of action and effect on cardiovascular events. Current hypertension reports 15:514–521
Kudumula N, Prasad YR (2014) Development and validation of RP-HPLC method for the simultaneous estimation of chlorthalidone and cilnidipine in bulk and combined tablet dosage form. Pharmacophore 5:442–450
Dagariya RK, Jat RK (2017) Method development and validation of irbesartan, chlorthalidone and cilnidipine in their combined tablet dosage form by high performance liquid chromatography. Journal of Drug Delivery and Therapeutics 7:88–96
Chinthala K, Krishnamurthy M, Kumar P (2016) Stability indicating method development and validation for the simultaneous estimation of olmesartan, chlorthalidone and cilnidipine in bulk and pharmaceutical dosage form by using RP-HPLC. International Journal of Pharmacy 6:149–160
Patel MP, Patel KP, Patel DB (2016) Development and validation of analytical method for simultaneous estimation of cilnidipine, chlorthalidone and telmisartan in tablet dosage form. World Journal of Pharmacy and Pharmaceutical Sciences 5:1228–1241
Nayak PR, Chaudhary AB, Rahevar NM (2016) RP-HPLC method development and validation for simultaneous estimation of chlorthalidone, cilnidipine and olmesartan in tablet dosage form. World Journal of Pharmacy and Pharmaceutical Sciences 5:1498–1508
Parmar KE, Patel ND (2013) Stability indicating RP-HPLC method for simultaneous determination of telmisartan and chlorthalidone in bulk and pharmaceutical dosage form. International Journal of PharmTech Research 5:1728–1735
Mourya ND, Prajapati Y, Sakhreliya B (2017) Development and validation of RP-HPLC method for simultaneous estimation of chlorthalidone, cilnidipine and irbesartan in their combined marketed dosage form. Journal of Pharmaceutical and BioSciences 7:193–199
Sawale V, Dangre P, Dhabarde D (2015) Development and validation of RP-HPLC method for the simulteneous estimation of olmesartan medoxomil and chlorthalidone in tablet dosage form. International Journal of Pharmacy and Pharmaceutical Sciences 7:266–269
Sharma A, Mishra A, Sharma S (2016) Stability indicating simultaneous validation of telmisartan, cilnidipine and chlorthalidone with forced degradation behavior study by RP-HPLC in tablet dosage form. International Journal of Chemistry and Pharmaceutical Sciences 7:6–12
Charde MS, Welankiwar AS, Chakole RD (2014) Development of validated RP-HPLC method for the simultaneous estimation of atenolol and chlorthalidone in combine tablet dosage form. International Journal of Advanced Pharmaceutical Sciences 3:6–18
Lavanya K, Srinivasa VR, Sunitha P, Rama KPS (2017) Analytical RP- HPLC method development and validation for simultaneous estimation of azilsartan medoxomil and chlorthalidone in pharmaceutical dosage form. American Journal of PharmTech Research 7:599–606
Yunooos M, Sankar DG (2015) Stability indicating reverse phase LC method development and validation for simultaneous estimation of metoprolol succinate and chlorthalidone in combined tablet dosage form. Der Pharmacia Lettre 7:162–172
Parmar KE, Mehta RS, Patel ND, Parmar KE (2013) Development and validation of HPTLC method for simultaneous determination of telmisartan and chlorthalidone in bulk and pharmaceutical dosage form. International Journal of Pharmacy and Pharmaceutical Sciences 5:420–425
Youssef RM, Maher HM, El-Kimary EI, Hassan EM, Barary MH (2013) Validated stability-indicating methods for the simultaneous determination of amiloride hydrochloride, atenolol, and chlorthalidone using HPTLC and HPLC with photodiode array detector. Journal of AOAC International 96:313–323
Sangle S, Deshpande P, Shinde N, Tayade V (2014) Development and validation of stability indicating HPTLC method for simultaneous determination of olmesartan medoxomil and chlorthalidone in combined tablet dosage forms. European Journal of Pharmaceutical and Medical Research 4:574–581
Sawale V, Dhabarde DM, Mahapatra DK (2017) Development and validation of UV- spectrophotometric method for simultaneous estimation of olmesartan medoxomil and chlorthalidone in bulk and tablet. Eurasian Journal of Analytical Chemistry 12:55–66
Abdullah NS, Hassan MA, Hassan RO (2017) Spectrophotometric determination of chlorthalidone in pharmaceutical formulations using different order derivative methods. Arabian Journal of Chemistry 10:S3426–S3433
Padmane SP, Jain ND, Ittadwar AM, Walde SP (2014) A derivative UV-spectrophotometric method for the simultaneous determination of metoprolol succinate and chlorthalidone in combined dose tablet formulation. International Journal of Analytical and Bioanalytical Chemistry 4:33–41
Niraimathi V, Jerad SA, Kumar SI (2013) UV-Spectrophotometric methods for the estimation of chlorthalidone in bulk and oral dosage form. Indo American Journal of Pharmaceutical Research 3:7160–7167
Charde M, Welankiwar A, Chakole R (2014) Simultaneous estimation of atenolol and chlorthalidone in combine tablet dosage form by absorption ratio method using UV-vis spectrophotometry. International Journal of Advanced Pharmaceutical Sciences 3:2320–2327
Parmar KE, Mehta RS (2013) First order derivative spectrophotometry method for simultaneous determination of telmisartan and chlorthalidone in bulk and in pharmaceutical dosage form. International Research Journal of Pharmacy 4:224–228
Patel SN, Hinge MA, Bhanushali VM (2015) Development and validation of an UV spectrophotometric method for simultaneous determination of cilnidipine and chlorthalidone. Journal of Pharmacy Research 9:41–45
Parikh P, Sahoo U, Zanvar A, Seth AK (2013) Derivative spectrophotometric method for simultaneous estimation of chlorthalidone and olmesartan medoxomil in their tablet dosage form. An International Journal of Pharmaceutical Sciences 4:111–123
Ingle SU, Patil PA, Kulkarni VC, Patil SV, Salunke PA, Wagh RS (2010) Development and validation of UV spectrophotometric method for chlorthalidone in bulk and pharmaceutical dosage forms. International Journal of ChemTech Research 3:958–963
Patel S, Patel D (2013) Simultaneous determination of metoprolol succinate and chlorthalidone by UV-spectrophotometric method. Pharmagene 1:39–43
Ebeid WM, Elkady EF, El-Zaher AA, El-Bagary RI, Patonay G (2014) Spectrophotometric and spectrofluorimetric studies on azilsartan medoxomil and chlorthalidone to be utilized in their determination in pharmaceuticals. Analytical Chemistry Insights 9:33
Akifulhaque M, Nivedita G, Prashanthkumar K, Pradeepkumar T, Hasan SH, Diwan VP (2012) Simultaneous estimation of atenolol and chlorthalidone as bulk and in tablet dosage form using UV-spectrophotometry. Journal of Pharmaceutical and Biological Sciences 1:20–23
Ferraro MC, Castellano PM, Kaufman TS (2013) Chemometrics-assisted simultaneous determination of atenolol and chlorthalidone in synthetic binary mixtures and pharmaceutical dosage forms. Analytical and Bioanalytical Chemistry 377:1159–1164
Raval HR, Patel DM, Patel CN (2011) Estimation of metoprolol tartrate and chlorthalidone in combined dosage form by UV-spectrophotometric methods. Research Journal of Pharmacy and Technology 4:1204–1206
Vetuschi C, Ragno G (1990) Fourth UV derivative spectrophotometry for the simultaneous assay of atenolol and chlorthalidone in pharmaceuticals. International Journal of Pharmaceutics 65:177–181
Al Azzam K, Elbashir AA, Elbashir MA, Saad B, Abdul Hamid S (2009) Simultaneous determination of atenolol and chlorthalidone in pharmaceutical preparations by capillary-zone electrophoresis. Analytical Letters 42:1458–1470
Al Azzam KM, Saad B, Aboul-Enein HY (2010) Simultaneous determination of atenolol, chlorthalidone and amiloride in pharmaceutical preparations by capillary zone electrophoresis with ultraviolet detection. Biomedical Chromatography 24:977–981
Balesteros MR, Faria AF, de Oliveira MA (2007) Determination of losartan associated with chlorthalidone or hydrochlorothiazide in capsules by capillary zone electrophoresis. Journal of Brazilian Chemical Society 18:554–558
Fleuren HLJ, Van Ginneken CAM, Van Rossum JM (1979) Differential potentiometric method for determining dissociation constants of very slightly water-soluble drugs applied to the sulfonamide diuretic chlorthalidone. Journal of Pharmaceutical Sciences 68:1056–1058
Elbashir AA, Ahmed AA, Ali Ahmed SM, Aboul-Enein HY (2012) 1, 2-naphthoquinone-4-sulfonate sodium salt (NQS) as an analytical reagent for the determination of pharmaceutical amine by spectrophotometry. Applied Spectroscopy Reviews 47:219–232
AAT Bioquest, Inc. https://www.aatbio.com/resources/buffer-preparations-and recipes/carbonate-bicarbonate-buffer-ph-9-2-to-10-6. .
Job P (1964) Advanced physiochemical experiments. Analytical Chemistry 16:54
Renny JS, Tomasevich LL, Tallmadge EH, Collum DB (2013) Method of continuous variations: applications of Job plots to the molecular associations in organometallic chemistry. Angewandte Chemie International Edition 52:11998–12013
ICH (2005). ICH Topic Q2 (R1) Validation of analytical procedures: text and methodology.
Al-Araji RR, Mashkour MS, Al-Mulla EAJ (2017) Spectrophotometric determination of vitamin folic acid B9 in some drugs using 1, 2-naphthoquine-4-sulphonate (NQS). Nano Biomedicine and Engineering 9:208–213
Gouda AA, Hashem H, Hassan W (2012) Spectophotometric methods for determination of cefdinir in pharmaceutical formulations via derivatization with 1, 2-naphthoquinone-4-sulfonate and 4-chloro-7-nitrobenzo-2-oxa-1, 3-diazole. Drug Testing and Analysis 4:991–1000
Acknowledgements
The authors are thankful to the Principal of R. C. Patel Institute of Pharmaceutical Education and Research, Shirpur (MS), India, for providing essential laboratory facilities to carry out this work.
Funding
Not applicable
Author information
Authors and Affiliations
Contributions
SRC carried out the proposed research work and organized the preliminary draft of the manuscript. AAS gave technical contribution for completing the research work. The authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chaudhari, S.R., Shirkhedkar, A.A. Exploration of 1, 2-naphthoquinone-4-sulfonate derivatizing reagent for determination of chlorthalidone in tablets: a spectrophotometric investigation. Futur J Pharm Sci 7, 27 (2021). https://doi.org/10.1186/s43094-021-00180-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43094-021-00180-z