Abstract
Introduction/Purpose
Obesity increases significantly every year worldwide. Since 1980, the prevalence of individuals with obesity has practically doubled. Obesity plays an important role in the pathophysiology of diseases that arise from a complex interaction of nutritional, genetic, and metabolic factors, characterizing a chronic inflammatory state. This study aimed to verify the systemic inflammatory response through the analysis of IGF-1, IL-23, and resistin levels and the lipid profile in severely obese women undergoing surgery for obesity and weight-related diseases.
Materials and Methods
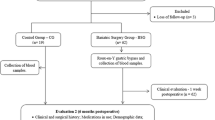
This randomized controlled clinical trial includes female patients clinically diagnosed with severe obesity with an indication for bariatric surgery.
Results
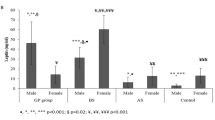
In the initial evaluation, no significant difference was observed between the control (CG) and bariatric surgery (BSG) groups. The weight, BMI, systolic and diastolic blood pressures, total cholesterol, LDL, HDL, total non-HDL cholesterol, and glucose in BSG patients showed a significant change after surgery. Pre- and post-surgery levels of resistin, IGF-1, and IL-23 showed a significant difference in the BSG group, but only IL-23 was changed after 6 months in the CG.
Conclusion
The results of this study confirmed that weight loss induced by surgery for obesity and weight-related diseases improved the lipid profile and reduced the chronic inflammatory status in women with severe obesity.
Graphical abstract






Similar content being viewed by others
References
Smith KB, Smith MS. Obesity statistics. Prim Care. 2016;43(1):121–35.
Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech Rep Ser. 2000;894:i-xii, 1–253.
WHO (World Health Organization). Obesity and overweight. Key facts. Fact sheet. 2021. Available from: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight. Accessed in: 03 Aug. 2021.
Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults--The Evidence Report. National Institutes of Health. Obes Res. 1998;6 Suppl 2:51S-209S. Erratum in: Obes Res 1998 Nov;6(6):464.
Hu FB. Obesity epidemiology. Oxford: Oxford University Press; 2008. p. 498.
Ng M, Fleming T, Robinson M, et al. Global, regional, and national prevalence of overweight and obesity in children and adults 1980–2013: a systematic analysis. Lancet. 2014;384(9945):766–81.
Mapa da Obesidade. Associação Brasileira para o Estudo da Obesidade e da Síndrome Metabólica (Abeso). São Paulo; 2020. Available from: https://abeso.org.br/. Brazilian Portuguese. Accessed 2021 Aug 03
Vigitel Brazil 2019: surveillance of risk and protective factors for chronic diseases by telephone survey: estimates of frequency and sociodemographic distribution of risk and protective factors for chronic diseases in the capitals of the 26 Brazilian states and the Federal District in 2019. World Wide Web: Available from: http://bvsms.saude.gov.br/bvs/publicacoes/vigitel_brasil_2019_vigilancia_fatores_risco.pdf. Accessed 31 Aug 2021.
Poirier P. Adiposity and cardiovascular disease: are we using the right definition of obesity? Eur Heart J. 2007;28(17):2047–8.
Karczewski J, Śledzińska E, Baturo A, et al. Obesity, and inflammation. Eur Cytokine Netw. 2018;29(3):83–94.
Wu H, Ballantyne CM. Metabolic inflammation and insulin resistance in obesity. Circ Res. 2020;126(11):1549–64.
Chrysant SG. Pathophysiology and treatment of obesity-related hypertension. J Clin Hypertens (Greenwich). 2019;21(5):555–9.
Banerjee S, Talukdar I, Banerjee A, et al. Type II diabetes mellitus and obesity: common links, existing therapeutics and future developments. J Biosci. 2019;44(6):150.
Russel SM, Valle V, Spagni G, et al. Physiologic mechanisms of type II diabetes mellitus remission following bariatric surgery: a meta-analysis and clinical implications. J Gastrointest Surg. 2020;24(3):728–41.
Meurling IJ, Shea DO, Garvey JF. Obesity and sleep: a growing concern. Curr Opin Pulm Med. 2019;25(6):602–8.
Perez EA, Oliveira LVF, Freitas WR Jr, et al. Prevalence and severity of syndrome Z in women with metabolic syndrome on waiting list for bariatric surgery: a cross-sectional study. Diabetol Metab Syndr. 2017;20(9):72.
Scotece M, Conde J, López V, et al. Adiponectin and leptin: new targets in inflammation. Basic Clin Pharmacol Toxicol. 2014;114(1):97–102.
Moussa O, Ardissino M, Muttoni S, et al. Long-term incidence and outcomes of obesity-related peripheral vascular disease after bariatric surgery. Langenbecks Arch Surg. 2021;406(4):1029–36.
Carmona-Maurici J, Cuello E, Sánchez E, et al. Impact of bariatric surgery on subclinical atherosclerosis in patients with morbid obesity. Surg Obes Relat Dis. 2020;16(10):1419–28.
Farias G, Netto BDM, Boritza K, et al. Impact of weight loss on inflammation state and endothelial markers among individuals with extreme obesity after gastric bypass surgery: a 2-year follow-up study. Obes Surg. 2020;30(5):1881–90.
Carmona-Maurici J, Cuello E, Ricart-Jané D, et al. Effect of bariatric surgery on inflammation and endothelial dysfunction as processes underlying subclinical atherosclerosis in morbid obesity. Surg Obes Relat Dis. 2020;16(12):1961–70.
Koenen M, Hill MA, Cohen P, et al. Obesity, adipose tissue and vascular dysfunction. Circ Res. 2021;128(7):951–68.
van den Berge M, van der Beek AJ, Türkeli R, et al. Work-related physical and psychosocial risk factors cluster with obesity, smoking and physical inactivity. Int Arch Occup Environ Health. 2021;94(4):741–50.
Wang X, Sun H, Ma B, et al. Insulin-like growth factor 1 related to chronic low-grade inflammation in patients with obesity and early change of its levels after laparoscopic sleeve gastrectomy. Obes Surg. 2020;30(9):3326–32.
Paroutoglou K, Papadavid E, Christodoulatos GS, et al. Deciphering the association between psoriasis and obesity: current evidence and treatment considerations. Curr Obes Rep. 2020;9(3):165–78.
Liu Y, Jin J, Chen Y, et al. Integrative analyses of biomarkers and pathways for adipose tissue after bariatric surgery. Adipocyte. 2020;9(1):384–400.
Min T, Prior SL, Dunseath G, et al. Temporal effects of bariatric surgery on adipokines, inflammation and oxidative stress in subjects with impaired glucose homeostasis at 4 years of follow-up. Obes Surg. 2020;30(5):1712–8.
Chiappetta S, Schaack HM, Wölnerhannsen B, et al. The impact of obesity and metabolic surgery on chronic inflammation. Obes Surg. 2018;28(10):3028–40.
Tripathi D, Kant S, Pandey S, et al. Resistin in metabolism, inflammation, and disease. FEBS J. 2020;287(15):3141–9.
Biobaku F, Ghanim H, Monte SV, et al. Bariatric surgery remission of inflammation, cardiometabolic benefits, and common adverse effects. J Endocr Soc. 2020;4(9):bvaa049.
Tchang BG, Saunders KH, Igel LI. Best practices in the management of overweight and obesity. Med Clin North Am. 2021;105(1):149–74.
American Diabetes Association. 8. Obesity Management for the treatment of type 2 diabetes: standards of medical care in diabetes-2021. Diabetes Care. 2021;44(Suppl 1):S100–10.
Karlsson J, Galavazi M, Jansson S, et al. Effects on body weight, eating behavior, and quality of life of a low-energy diet combined with behavioral group treatment of persons with class II or III obesity: a 2-year pilot study. Obes Sci Pract. 2020;7(1):4–13.
LeBlanc ES, Patnode CD, Webber EM, et al. Behavioral and pharmacotherapy weight loss interventions to prevent obesity-related morbidity and mortality in adults: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2018;320(11):1172–91.
De Luca M, Angrisani L, Himpens J, et al. Indications for surgery for obesity and weight-related diseases: position statements from the international federation for the surgery of obesity and metabolic disorders (IFSO). Obes Surg. 2016;26(8):1659–96.
Dilektasli E, Demir B. Definitions and current indications for obesity and metabolic surgery. Ann Laparosc Endosc Surg. 2021;20(6):8.
Stahl JM, Malhotra S. Obesity surgery indications and contraindications. 2020 Jul 31. In: StatPearls. Treasure Island: StatPearls Publishing; 2021.
Sümer A. Definition of obesity and current indications for obesity surgery. Laparosc Endosc Surg Sci. 2016;23(3):56–62.
Chan AW, Tetzlaff JM, Altman DG, et al. SPIRIT 2013 statement: defining standard protocol items for clinical trials. Ann Intern Med. 2013;158(3):200–7.
Buchwald H, Consensus Conference Panel. Consensus conference statement bariatric surgery for morbid obesity: health implications for patients, health professionals, and third-party payers. Surg Obes Relat Dis. 2005;1(3):371–81.
Ikramuddin S, Kendrick ML, Kellogg TA, et al. Open and laparoscopic Roux-en-Y gastric bypass: our techniques. J Gastrointest Surg. 2007;11(2):217–28.
Freitas WR Jr, Oliveira LVF, Perez EA, et al. Systemic inflammation in severe obese patients undergoing surgery for obesity and weight-related diseases. Obes Surg. 2018;28(7):1931–42.
Arismendi E, Rivas E, Agustí A, et al. The systemic inflammome of severe obesity before and after bariatric surgery. PLoS One. 2014;9(9):e107859.
Kawai T, Autieri MV, Scalia R. Adipose tissue inflammation and metabolic dysfunction in obesity. Am J Physiol Cell Physiol. 2021;320(3):C375–91.
Tam CS, Redman LM. Adipose tissue inflammation and metabolic dysfunction: a clinical perspective. Horm Mol Biol Clin Invest. 2013;15(1):19–24.
Illán-Gómez F, Gonzálvez-Ortega M, Orea-Soler I, et al. Obesity and inflammation: change in adiponectin, C-reactive protein, tumour necrosis factor-alpha and interleukin-6 after bariatric surgery. Obes Surg. 2012;22(6):950–5.
Chiappetta S, Jamadar P, Stier C, et al. The role of C-reactive protein after surgery for obesity and metabolic disorders. Surg Obes Relat Dis. 2020;16(1):99–108.
de Lima-Junior JC, Virginio VWM, Moura FA, et al. Excess weight mediates changes in HDL pool that reduce cholesterol efflux capacity and increase antioxidant activity. Nutr Metab Cardiovasc Dis. 2020;30(2):254–64.
Buchwald H, Avidor Y, Braunwald E, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA. 2004;292(14):1724–37.
Malheiros C, Freitas JRW, Saleh M, et al. Bariatric surgery decreases the inflammatory response in the 6 months post-operatory. Obes Surg. 2009;19:1013.
Goktas Z, Moustaid-Moussa N, Shen CL, et al. Effects of bariatric surgery on adipokine-induced inflammation and insulin resistance. Front Endocrinol (Lausanne). 2013;10(4):69.
Farkhondeh T, Llorens S, Pourbagher-Shahri AM, et al. An overview of the role of adipokines in cardiometabolic diseases. Molecules. 2020;25(21):5218.
ParreñoCaparrós E, Illán Gómez F, Gonzálvez Ortega M, et al. Resistin in morbidly obese patients before and after gastric bypass surgery. Nutr Hosp. 2017;34(5):1333–7.
Rodríguez-López CP, González-Torres MC, Cruz-Bautista I, et al. Visceral obesity, skeletal muscle mass and resistin in metabolic syndrome development. Nutr Hosp. 2019;36(1):43–50.
Su KZ, Li YR, Zhang D, et al. Relation of circulating resistin to insulin resistance in type 2 diabetes and obesity: a systematic review and meta-analysis. Front Physiol. 2019;19(10):1399.
Jamaluddin MS, Weakley SM, Yao Q, et al. Resistin: functional roles and therapeutic considerations for cardiovascular disease. Br J Pharmacol. 2012;165(3):622–32.
Fontana A, Spadaro S, Copetti M, et al. Association between resistin levels and all-cause and cardiovascular mortality: a new study and a systematic review and meta-analysis. PLoS One. 2015;10(3):e0120419.
Wang YY, Hung AC, Lo S, et al. Adipocytokines visfatin and resistin in breast cancer: Clinical relevance, biological mechanisms, and therapeutic potential. Cancer Lett. 2021;1(498):229–39.
Miethe C, Zamora M, Torres L, et al. Characterizing the differential physiological effects of adipocytokines visfatin and resistin in liver cancer cells. Horm Mol Biol Clin Investig. 2019;38(2):/j/hmbci.2019.38.issue-2/hmbci-2018-0068/hmbci-2018-0068.xml.
Miethe C, Torres L, Beristain J, et al. The role of visfatin and resistin in an in vitro model of obesity-induced invasive liver cancer. Can J Physiol Pharmacol. 2020;23:1–8.
Yang G, Fan W, Luo B, et al. Circulating resistin levels and risk of colorectal cancer: a meta-analysis. Biomed Res Int. 2016;2016:7367485.
Zhang M, Yan L, Wang GJ, et al. Resistin effects on pancreatic cancer progression and chemoresistance are mediated through its receptors CAP1 and TLR4. J Cell Physiol. 2019;234(6):9457–66.
Qiu L, Zhang GF, Yu L, et al. Novel oncogenic and chemoresistance-inducing functions of resistin in ovarian cancer cells require miRNAs-mediated induction of epithelial-to-mesenchymal transition. Sci Rep. 2018;8(1):12522.
Gong WJ, Zheng W, Xiao L, et al. Circulating resistin levels and obesity-related cancer risk: a meta-analysis. Oncotarget. 2016;7(36):57694–704.
Edwards C, Hindle AK, Fu S, et al. Downregulation of leptin and resistin expression in blood following bariatric surgery. Surg Endosc. 2011;25(6):1962–8.
Martinelli CE Jr, Custódio RJ, Aguiar-Oliveira MH. Fisiologia do Eixo GH-Sistema IGF. Arq Bras Endrocrinol Metab. 2008;52(5):717–25.
Juiz-Valiña P, Pena-Bello L, Cordido M, et al. Altered GH-IGF-1 axis in severe obese subjects is reversed after bariatric surgery-induced weight loss and related with low-grade chronic inflammation. J Clin Med. 2020;9(8):2614.
Pellitero S, Granada ML, Martínez E, et al. IGF1 modifications after bariatric surgery in morbidly obese patients: potential implications of nutritional status according to specific surgical technique. Eur J Endocrinol. 2013;169(5):695–703.
Pardina E, Ferrer R, Baena-Fustegueras JA, et al. The relationships between IGF-1 and CRP, NO, leptin, and adiponectin during weight loss in the morbidly obese. Obes Surg. 2010;20(5):623–32.
Higashi Y, Gautam S, Delafontaine P, et al. IGF-1 and cardiovascular disease. Growth Horm IGF Res. 2019;45:6–16.
Obradovic M, Zafirovic S, Soskic S, et al. Effects of IGF-1 on the Cardiovascular System. Curr Pharm Des. 2019;25(35):3715–25.
Dichtel LE, Corey KE, Misdraji J, et al. The association between igf-1 levels and the histologic severity of nonalcoholic fatty liver disease. Clin Transl Gastroenterol. 2017;8(1):e217.
Sádaba MC, Martín-Estal I, Puche JE, et al. Insulin-like growth factor 1 (IGF-1) therapy: Mitochondrial dysfunction and diseases. Biochim Biophys Acta. 2016;1862(7):1267–78.
Olleros Santos-Ruiz M, Sádaba MC, Martín-Estal I, et al. The single IGF-1 partial deficiency is responsible for mitochondrial dysfunction and is restored by IGF-1 replacement therapy. Growth Horm IGF Res. 2017;35:21–32.
Rasmussen MH, Juul A, Hilsted J. Effect of weight loss on free insulin-like growth factor-I in obese women with hyposomatotropism. Obesity (Silver Spring). 2007;15(4):879–86.
Weroha SJ, Haluska P. The insulin-like growth factor system in cancer. Endocrinol Metab Clin North Am. 2012;41(2):335–vi.
Sumarac-Dumanovic M, Stevanovic D, Ljubic A, et al. Increased activity of interleukin-23/interleukin-17 proinflammatory axis in obese women. Int J Obes (Lond). 2009;33(1):151–6.
Neurath MF. IL-23 in inflammatory bowel diseases and colon cancer. Cytokine Growth Factor Rev. 2019;45:1–8.
Yan J, Smyth MJ, Teng MWL. Interleukin (IL)-12 and IL-23 and their conflicting roles in cancer. Cold Spring Harb Perspect Biol. 2018;10(7):a028530.
Chyuan IT, Lai JH. New insights into the IL-12 and IL-23: from a molecular basis to clinical application in immune-mediated inflammation and cancers. Biochem Pharmacol. 2020;175:113928.
Ge W, Hu H, Cai W, et al. High-risk stage III colon cancer patients identified by a novel five-gene mutational signature are characterized by upregulation of IL-23A and gut bacterial translocation of the tumor microenvironment. Int J Cancer. 2020;146(7):2027–35.
Hirano T, Hirayama D, Wagatsuma K, et al. Immunological mechanisms in inflammation-associated colon carcinogenesis. Int J Mol Sci. 2020;21(9):3062.
Wu S, Rhee KJ, Albesiano E, et al. A human colonic commensal promotes colon tumorigenesis via activation of T helper type 17 T cell responses. Nat Med. 2009;15(9):1016–22.
Acknowledgements
The authors would like to acknowledge the technical infrastructure support provided by Department of Surgery of Santa Casa Medical School (Sao Paulo, Brazil).
Funding
ARTS, EAP, and ASS receives grants of Coordenaçao de Apoio ao Pessoal de Nível Superior (CAPES/PROSUP); JPRA receive grants of Fundação de Amparo a Pesquisa (FAPEG), Goiás (GO), Brazil; LVFO receive grants Research Productivity, modality PQ1D; process no. 312731/2018–3 of Conselho Nacional de Desenvolvimento Cientifico e Tecnologico (local acronym CNPq), Brazil.
Author information
Authors and Affiliations
Contributions
Conceived and designed of the study: LVFO, ARTS, WRFJ, CAM, and GI. Acquisition, statistical analysis, or interpretation of the data: EAP, ASS, VLSA, JPRA, MCO, LVFO, ALF, MEML, MCOJ, RPV, WJSP, and ALLB. Evaluation and implementation of bariatric surgery: ARTS, WRFJ, EJI, and CAM. Follow-up of surgical patients: MMS, ARTS, WRFJ, and CAM. Checking and interpretation of the data, drafting of the manuscript, and approved the submitted version of the manuscript: all authors.
Corresponding author
Ethics declarations
Ethical Approval
All procedures performed in this study involving human participants were in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki declaration and its later amendments. This research was approved by the Human Research Ethics 564 Committees of Nove de Julho University (UNINOVE; protocol number 565 220506/2009) and Irmandade da Santa Casa de Misericordia de Sao 566 Paulo, Brazil (protocol number 742.865/2014). This trial was registered at ClinicalTrials.gov (02409160).
Informed Consent
Informed consent was obtained from all individual participants included in this study.
Conflict of Interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Key Points
- Unfortunately, weight loss resulting from clinical treatment for obesity is hardly achieved and sustained.
- Weight loss induced by surgery for obesity and weight-related diseases improved the lipid profile.
- The chronic inflammatory profile observed in severely obese women is considerably reduced after weight loss induced by surgery for obesity and weight-related diseases.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
de Sousa, A.R.T., Freitas Junior, W.R., Perez, E.A. et al. Surgery for Obesity and Weight-Related Diseases Changes the Inflammatory Profile in Women with Severe Obesity: a Randomized Controlled Clinical Trial. OBES SURG 31, 5224–5236 (2021). https://doi.org/10.1007/s11695-021-05702-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-021-05702-5