Abstract
Background
Gastric plication is a minimally invasive bariatric surgical procedure, where the greater curvature is plicated inside the gastric lumen. Our aims were to analyze the effectiveness of gastric plication on the resolution of obesity, impaired glucose tolerance, and fatty liver in an experimental model of diet-induced obesity (DIO) and to evaluate changes in glycerol metabolism, a key substrate for adiposity and gluconeogenesis, in adipose tissue and the liver.
Methods
Male Wistar DIO rats (n = 58) were subjected to surgical (sham operation and gastric plication) or dietary interventions [fed a normal diet (ND) or high-fat diet (HFD) or pair-fed to the amount of food eaten by gastric-plicated animals]. The expression of aquaglyceroporins (AQPs) in epididymal (EWAT) and subcutaneous (SCWAT) fat and the liver was analyzed by real-time PCR and Western blot.
Results
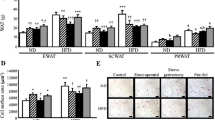
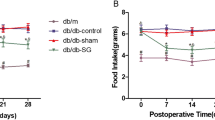
Gastric plication did not result in a significant weight loss in DIO rats, showing a modest reduction in whole-body adiposity and hepatic steatosis. However, gastric-plicated animals exhibited an improvement in basal glycemia and glucose clearance, without changes in hepatic gluconeogenic genes. DIO was associated with an increase in glycerol, higher AQP3 and AQP7 in EWAT and SCWAT, and a decrease in hepatic AQP9. Gastric plication downregulated AQP3 in both fat depots without changes in adipose AQP7 and hepatic AQP9.
Conclusion
Gastric plication results in a modest reduction in adiposity and hepatosteatosis but restores glycemia by downregulating AQP3, which entails lower efflux of glycerol from fat, lower plasma glycerol availability, and a reduced use of glycerol as a substrate for hepatic gluconeogenesis.






Similar content being viewed by others
Abbreviations
- Adipo-IR:
-
Adipocyte insulin resistance index
- AQP:
-
Aquaporin
- CSA:
-
Cell surface area
- DIO:
-
Diet-induced obesity
- EWAT:
-
Epididymal white adipose tissue
- EWL:
-
Excess weight loss
- FFA:
-
Free fatty acids
- GK:
-
Glycerol kinase
- HFD:
-
High-fat diet
- HOMA:
-
Homeostasis model assessment
- NAFLD:
-
Non-alcoholic fatty liver disease
- ND:
-
Normal diet
- PCK1:
-
Phosphoenolpyruvate carboxykinase 1
- PPARγ:
-
Peroxisome proliferator activator receptor γ
- PRWAT:
-
Perirenal white adipose tissue
- QUICKI:
-
Quantitative insulin sensitivity check index
- SCWAT:
-
Subcutaneous white adipose tissue
- SREBF1:
-
Sterol regulatory element-binding factor 1
- TG:
-
Triacylglycerol
References
Reshef L, Olswang Y, Cassuto H, et al. Glyceroneogenesis and the triglyceride/fatty acid cycle. J Biol Chem. 2003;278:30413–6.
Frühbeck G. Obesity: aquaporin enters the picture. Nature. 2005;438:436–7.
Rodríguez A, Catalán V, Gómez-Ambrosi J, et al. Aquaglyceroporins serve as metabolic gateways in adiposity and insulin resistance control. Cell Cycle. 2011;10:1548–56.
Hibuse T, Maeda N, Funahashi T, et al. Aquaporin 7 deficiency is associated with development of obesity through activation of adipose glycerol kinase. Proc Natl Acad Sci U S A. 2005;102:10993–8.
Hara-Chikuma M, Sohara E, Rai T, et al. Progressive adipocyte hypertrophy in aquaporin-7-deficient mice: adipocyte glycerol permeability as a novel regulator of fat accumulation. J Biol Chem. 2005;280:15493–6.
Rodríguez A, Catalán V, Gómez-Ambrosi J, et al. Insulin- and leptin-mediated control of aquaglyceroporins in human adipocytes and hepatocytes is mediated via the PI3K/Akt/mTOR signaling cascade. J Clin Endocrinol Metab. 2011;96:E586–97.
Laforenza U, Scaffino MF, Gastaldi G. Aquaporin-10 represents an alternative pathway for glycerol efflux from human adipocytes. PLoS One. 2013;8:e54474.
Madeira A, Fernandez-Veledo S, Camps M, et al. Human aquaporin-11 is a water and glycerol channel and localizes in the vicinity of lipid droplets in human adipocytes. Obesity. 2014;22:2010–7.
Madeira A, Mosca AF, Moura TF, et al. Aquaporin-5 is expressed in adipocytes with implications in adipose differentiation. IUBMB Life. 2015;67:54–60.
Peroni O, Large V, Beylot M. Measuring gluconeogenesis with [2-13C]glycerol and mass isotopomer distribution analysis of glucose. Am J Phys. 1995;269:E516–23.
Rojek AM, Skowronski MT, Fuchtbauer EM, et al. Defective glycerol metabolism in aquaporin 9 (AQP9) knockout mice. Proc Natl Acad Sci U S A. 2007;104:3609–14.
Jelen S, Wacker S, Aponte-Santamaria C, et al. Aquaporin-9 protein is the primary route of hepatocyte glycerol uptake for glycerol gluconeogenesis in mice. J Biol Chem. 2011;286:44319–25.
Rodríguez A, Gena P, Méndez-Giménez L, et al. Reduced hepatic aquaporin-9 and glycerol permeability are related to insulin resistance in non-alcoholic fatty liver disease. Int J Obes. 2014;38:1213–20.
Ma T, Song Y, Yang B, et al. Nephrogenic diabetes insipidus in mice lacking aquaporin-3 water channels. Proc Natl Acad Sci U S A. 2000;97:4386–91.
Marrades MP, Milagro FI, Martínez JA, et al. Differential expression of aquaporin 7 in adipose tissue of lean and obese high fat consumers. Biochem Biophys Res Commun. 2006;339:785–9.
Prudente S, Flex E, Morini E, et al. A functional variant of the adipocyte glycerol channel aquaporin 7 gene is associated with obesity and related metabolic abnormalities. Diabetes. 2007;56:1468–74.
Ceperuelo-Mallafré V, Miranda M, Chacón MR, et al. Adipose tissue expression of the glycerol channel aquaporin-7 gene is altered in severe obesity but not in type 2 diabetes. J Clin Endocrinol Metab. 2007;92:3640–5.
Catalán V, Gómez-Ambrosi J, Rotellar F, et al. Validation of endogenous control genes in human adipose tissue: relevance to obesity and obesity-associated type 2 diabetes mellitus. Horm Metab Res. 2007;39:495–500.
Gena P, Mastrodonato M, Portincasa P, et al. Liver glycerol permeability and aquaporin-9 are dysregulated in a murine model of non-alcoholic fatty liver disease. PLoS One. 2013;8:e78139.
Wakayama Y, Hirako S, Ogawa T, et al. Upregulated expression of AQP7 in the skeletal muscles of obese ob/ob mice. Acta Histochem Cytochem. 2014;47:27–33.
Catalán V, Gómez-Ambrosi J, Pastor C, et al. Influence of morbid obesity and insulin resistance on gene expression levels of AQP7 in visceral adipose tissue and AQP9 in liver. Obes Surg. 2008;18:695–701.
Rodríguez A, Moreno NR, Balaguer I, et al. Leptin administration restores the altered adipose and hepatic expression of aquaglyceroporins improving the non-alcoholic fatty liver of ob/ob mice. Sci Rep. 2015;5:12067.
Méndez-Giménez L, Becerril S, Moncada R, et al. Sleeve gastrectomy reduces hepatic steatosis by improving the coordinated regulation of aquaglyceroporins in adipose tissue and liver in obese rats. Obes Surg. 2015;25:1723–34.
Talebpour M, Motamedi SM, Talebpour A, et al. Twelve year experience of laparoscopic gastric plication in morbid obesity: development of the technique and patient outcomes. Ann Surg Innov Res. 2012;6:7.
Ramos A, Galvao Neto M, Galvao M, et al. Laparoscopic greater curvature plication: initial results of an alternative restrictive bariatric procedure. Obes Surg. 2010;20:913–8.
Brethauer SA, Harris JL, Kroh M, et al. Laparoscopic gastric plication for treatment of severe obesity. Surg Obes Relat Dis. 2011;7:15–22.
Niazi M, Maleki AR, Talebpour M. Short-term outcomes of laparoscopic gastric plication in morbidly obese patients: importance of postoperative follow-up. Obes Surg. 2013;23:87–92.
Verdi D, Prevedello L, Albanese A, et al. Laparoscopic gastric plication (LGCP) vs sleeve gastrectomy (LSG): a single institution experience. Obes Surg. 2015;25:1653–7.
Fusco PE, Poggetti RS, Younes RN, et al. Evaluation of gastric greater curvature invagination for weight loss in rats. Obes Surg. 2006;16:172–7.
Guimarães M, Nora M, Ferreira T, et al. Sleeve gastrectomy and gastric plication in the rat result in weight loss with different endocrine profiles. Obes Surg. 2013;23:710–7.
Kourkoulos M, Giorgakis E, Kokkinos C, et al. Laparoscopic gastric plication for the treatment of morbid obesity: a review. Minim Invasive Surg. 2012;2012:696348.
Darido E, Moore JR. Comparison of gastric fundus invagination and gastric greater curvature plication for weight loss in a rat model of diet-induced obesity. Obes Surg. 2014;24:897–902.
Martín M, Burrell MA, Gómez-Ambrosi J, et al. Short- and long-term changes in gastric morphology and histopathology following sleeve gastrectomy in diet-induced obese rats. Obes Surg. 2012;22:634–40.
Becerril S, Rodríguez A, Catalán V, et al. Deletion of inducible nitric-oxide synthase in leptin-deficient mice improves brown adipose tissue function. PLoS One. 2010;5:e10962.
Zhang J, Zhao Y, Xu C, et al. Association between serum free fatty acid levels and nonalcoholic fatty liver disease: a cross-sectional study. Sci Rep. 2014;4:5832.
Kishida K, Shimomura I, Nishizawa H, et al. Enhancement of the aquaporin adipose gene expression by a peroxisome proliferator-activated receptor γ. J Biol Chem. 2001;276:48572–9.
Jiang YJ, Kim P, Lu YF, et al. PPARgamma activators stimulate aquaporin 3 expression in keratinocytes/epidermis. Exp Dermatol. 2011;20:595–9.
Lebeck J, Cheema MU, Skowronski MT, et al. Hepatic AQP9 expression in male rats is reduced in response to PPARalpha agonist treatment. Am J Physiol Gastrointest Liver Physiol. 2015;308:G198–205.
Frühbeck G, Toplak H, Woodward E, et al. Obesity: the gateway to ill health—an EASO position statement on a rising public health, clinical and scientific challenge in Europe. Obes Facts. 2013;6:117–20.
Frühbeck G. Bariatric and metabolic surgery: a shift in eligibility and success criteria. Nat Rev Endocrinol. 2015;11:465–77.
Rodríguez A, Gómez-Ambrosi J, Catalán V, et al. Acylated and desacyl ghrelin stimulate lipid accumulation in human visceral adipocytes. Int J Obes. 2009;33:541–52.
Ivano FH, Silva Lde M, Seniski GG, et al. Comparison of ghrelin plasma levels between pre and postoperative period in patients submitted to gastric plication associated with fundoplication. Arq Bras Cir Dig. 2013;26(Suppl 1):8–12.
Page AJ, Slattery JA, Milte C, et al. Ghrelin selectively reduces mechanosensitivity of upper gastrointestinal vagal afferents. Am J Physiol Gastrointest Liver Physiol. 2007;292:G1376–84.
Kishida K, Kuriyama H, Funahashi T, et al. Aquaporin adipose, a putative glycerol channel in adipocytes. J Biol Chem. 2000;275:20896–902.
Yasui H, Kubota M, Iguchi K, et al. Membrane trafficking of aquaporin 3 induced by epinephrine. Biochem Biophys Res Commun. 2008;373:613–7.
Calamita G, Gena P, Ferri D, et al. Biophysical assessment of aquaporin-9 as principal facilitative pathway in mouse liver import of glucogenetic glycerol. Biol Cell. 2012;104:342–51.
Tardelli M, Moreno-Viedma V, Zeyda M, et al. Adiponectin regulates aquaglyceroporin expression in hepatic stellate cells altering their functional state. J Gastroenterol Hepatol. 2016; doi:10.1111/jgh.13415.
Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of non-alcoholic fatty liver disease: practice guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology. 2012;55:2005–23.
European Association for the Study of the Liver (EASL), European Association for the Study of Diabetes (EASD), European Association for the Study of Obesity (EASO). EASL-EASD-EASO clinical practice guidelines for the management of non-alcoholic fatty liver disease. J Hepatol. 2016;64:1388–402.
Utzschneider KM, Kahn SE. Review: the role of insulin resistance in nonalcoholic fatty liver disease. J Clin Endocrinol Metab. 2006;91:4753–61.
Dixon JB, Bhathal PS, O’Brien PE. Weight loss and non-alcoholic fatty liver disease: falls in gamma-glutamyl transferase concentrations are associated with histologic improvement. Obes Surg. 2006;16:1278–86.
Burza MA, Romeo S, Kotronen A, et al. Long-term effect of bariatric surgery on liver enzymes in the Swedish obese subjects (SOS) study. PLoS One. 2013;8:e60495.
Bower G, Athanasiou T, Isla AM, et al. Bariatric surgery and nonalcoholic fatty liver disease. Eur J Gastroenterol Hepatol. 2015;27:755–68.
Kubota N, Kubota T, Kajiwara E, et al. Differential hepatic distribution of insulin receptor substrates causes selective insulin resistance in diabetes and obesity. Nat Commun. 2016;7:12977.
Froylich D, Corcelles R, Daigle C, et al. Effect of Roux-en-Y gastric bypass and sleeve gastrectomy on nonalcoholic fatty liver disease: a comparative study. Surg Obes Relat Dis. 2016;12:127–31.
Moncada R, Becerril S, Rodríguez A, et al. Sleeve gastrectomy reduces body weight and improves metabolic profile also in obesity-prone rats. Obes Surg. 2016;26:1537–48.
Acknowledgements
We gratefully acknowledge the valuable collaboration of all the staff of the breeding house of the University of Navarra.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This article does not contain any studies with human participants.
All applicable institutional and national guidelines for the care and use of animals were followed. All experimental procedures conformed to the European guidelines for the care and use of laboratory animals (directive 2010/63/EU) and were approved by the Ethical Committee for Animal Experimentation of the University of Navarra (049/10).
Conflict of Interest
The authors declare that they have no conflict of interest.
Funding
This work was supported by Fondo de Investigación Sanitaria-FEDER (FIS PI13/01430 and PI16/01217) from the Instituto de Salud Carlos III, by the Department of Health of the Gobierno de Navarra (61/2014), and by the Plan de Investigación de la Universidad de Navarra (project PIUNA 2011-14). CIBEROBN is an initiative of the Instituto de Salud Carlos III, Spain.
Rights and permissions
About this article
Cite this article
Méndez-Giménez, L., Becerril, S., Moncada, R. et al. Gastric Plication Improves Glycemia Partly by Restoring the Altered Expression of Aquaglyceroporins in Adipose Tissue and the Liver in Obese Rats. OBES SURG 27, 1763–1774 (2017). https://doi.org/10.1007/s11695-016-2532-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-016-2532-2