Abstract
Background
Glycerol production and its efflux from adipocytes to the liver are key to modulate lipid and glucose homeostasis. Aquaporin 7 (AQP7) is an aquaglyceroporin that acts as the adipose glycerol channel, whereas aquaporin 9 (AQP9) is the specific channel operating in the liver. The aim of the present work was to evaluate the effect of obesity and type 2 diabetes mellitus (T2DM) on gene expression levels of AQP7 in visceral adipose tissue (VAT) and AQP9 in liver.
Methods
VAT and liver biopsies obtained from 20 women were used in the study. Patients were classified as lean or obese with the last group being further subclassified as normoglycemic (NG), patients with impaired glucose tolerance (IGT), or with T2DM. Anthropometric measurements as well as circulating metabolites, hormones, and adipokines were determined. Real-time polymerase chain reaction analyses were performed to quantify transcript levels of AQP7 in VAT and AQP9 in the liver.
Results
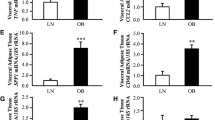
Gene expression levels of AQP7 in VAT showed a tendency toward an increase (P = 0.065) in obese patients (both NG and T2DM) compared to lean subjects. AQP9 showed a significant downregulation in the hepatic biopsies obtained from obese T2DM patients compared to obese NG and IGT patients (P = 0.028).
Conclusion
The tendency toward an elevation of mRNA expression of VAT AQP7 in obesity together with the decreased hepatic AQP9 expression observed in obese T2DM subjects suggests a potential role in facilitating glycerol release from adipose tissue and reducing glycerol entry into hepatocytes in obesity and T2DM, respectively.


Similar content being viewed by others
Abbreviations
- BF:
-
body fat percentage
- BMI:
-
body mass index
- AQP7:
-
aquaporin 7
- AQP9:
-
aquaporin 9
- NG:
-
normoglycemic
- OGTT:
-
oral glucose tolerance test
- T2DM:
-
type 2 diabetes mellitus
- VAT:
-
visceral adipose tissue
References
Frühbeck G, Gómez-Ambrosi J, Muruzábal FJ, et al. The adipocyte: a model for integration of endocrine and metabolic signaling in energy metabolism regulation. Am J Physiol Endocrinol Metab. 2001;280:E827–47.
Frühbeck G, Gómez-Ambrosi J. Control of body weight: a physiologic and transgenic perspective. Diabetologia. 2003;46:143–72.
Duncan RE, Ahmadian M, Jaworski K, et al. Regulation of lipolysis in adipocytes. Annu Rev Nutr. 2007;27:79–101.
Landau BR, Wahren J, Previs SF, et al. Glycerol production and utilization in humans: sites and quantitation. Am J Physiol. 1996;271:E1110–7.
Reshef L, Olswang Y, Cassuto H, et al. Glyceroneogenesis and the triglyceride/fatty acid cycle. J Biol Chem. 2003;278:30413–6.
Kuriyama H, Shimomura I, Kishida K, et al. Coordinated regulation of fat-specific and liver-specific glycerol channels, aquaporin adipose and aquaporin 9. Diabetes. 2002;51:2915–21.
Denker BM, Smith BL, Kuhajda FP, et al. Identification, purification, and partial characterization of a novel Mr 28,000 integral membrane protein from erythrocytes and renal tubules. J Biol Chem. 1988;263:15634–42.
King LS, Kozono D, Agre P. From structure to disease: the evolving tale of aquaporin biology. Nat Rev Mol Cell Biol. 2004;5:687–98.
Rodríguez A, Catalán V, Gómez-Ambrosi J, et al. Role of aquaporin-7 in the pathophysiological control of fat accumulation in mice. FEBS Lett. 2006;580:4771–6.
Kuriyama H, Kawamoto S, Ishida N, et al. Molecular cloning and expression of a novel human aquaporin from adipose tissue with glycerol permeability. Biochem Biophys Res Commun. 1997;241:53–8.
Frühbeck G, Catalán V, Gómez-Ambrosi J, et al. Aquaporin-7 and glycerol permeability as novel obesity drug-target pathways. Trends Pharmacol Sci. 2006;27:345–7.
Hibuse T, Maeda N, Nagasawa A, et al. Aquaporins and glycerol metabolism. Biochim Biophys Acta. 2006;1758:1004–11.
Frühbeck G. Obesity: aquaporin enters the picture. Nature. 2005;438:436–7.
Kishida K, Kuriyama H, Funahashi T, et al. Aquaporin adipose, a putative glycerol channel in adipocytes. J Biol Chem. 2000;275:20896–902.
Sjöholm K, Palming J, Olofsson LE, et al. A microarray search for genes predominantly expressed in human omental adipocytes: adipose tissue as a major production site of serum amyloid A. J Clin Endocrinol Metab. 2005;90:2233–9.
Maeda N, Funahashi T, Hibuse T, et al. Adaptation to fasting by glycerol transport through aquaporin 7 in adipose tissue. Proc Natl Acad Sci USA. 2004;101:17801–6.
Hara-Chikuma M, Sohara E, Rai T, et al. Progressive adipocyte hypertrophy in aquaporin-7-deficient mice: adipocyte glycerol permeability as a novel regulator of fat accumulation. J Biol Chem. 2005;280:15493–6.
Hibuse T, Maeda N, Funahashi T, et al. Aquaporin 7 deficiency is associated with development of obesity through activation of adipose glycerol kinase. Proc Natl Acad Sci USA. 2005;102:10993–8.
Elkjaer M, Vajda Z, Nejsum LN, et al. Immunolocalization of AQP9 in liver, epididymis, testis, spleen, and brain. Biochem Biophys Res Commun. 2000;276:1118–28.
Rojek AM, Skowronski MT, Fuchtbauer EM, et al. Defective glycerol metabolism in aquaporin 9 (AQP9) knockout mice. Proc Natl Acad Sci USA. 2007;104:3609–14.
Carbrey JM, Gorelick-Feldman DA, Kozono D, Praetorius J, Nielsen S, Agre P. Aquaglyceroporin AQP9: solute permeation and metabolic control of expression in liver. Proc Natl Acad Sci USA. 2003;100:2945–50.
Marrades MP, Milagro FI, Martínez JA, et al. Differential expression of aquaporin 7 in adipose tissue of lean and obese high fat consumers. Biochem Biophys Res Commun. 2006;339:785–9.
Prudente S, Flex E, Morini E, et al. A functional variant of the adipocyte glycerol channel aquaporin 7 gene is associated with obesity and related metabolic abnormalities. Diabetes. 2007;56:1468–74.
Ceperuelo-Mallafré V, Miranda M, Chacón MR, et al. Adipose tissue expression of the glycerol channel aquaporin-7 gene is altered in severe obesity but not in type 2 diabetes. J Clin Endocrinol Metab. 2007;92:3640–5.
Das SK, Roberts SB, Kehayias JJ, et al. Body composition assessment in extreme obesity and after massive weight loss induced by gastric bypass surgery. Am J Physiol Endocrinol Metab. 2003;284:E1080–8.
Matthews DR, Hosker JP, Rudenski AS, et al. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–9.
Katz A, Nambi SS, Mather K, et al. Quantitative insulin sensitivity check index: a simple, accurate method for assessing insulin sensitivity in humans. J Clin Endocrinol Metab. 2000;85:2402–10.
Gómez-Ambrosi J, Salvador J, Rotellar F, et al. Increased serum amyloid A concentrations in morbid obesity decrease after gastric bypass. Obes Surg. 2006;16:262–9.
Catalán V, Gómez-Ambrosi J, Ramírez B, et al. Proinflammatory cytokines in obesity: impact of type 2 diabetes mellitus and gastric bypass. Obes Surg. 2007;17:1464–74.
Gómez-Ambrosi J, Catalán V, Diez-Caballero A, et al. Gene expression profile of omental adipose tissue in human obesity. FASEB J. 2004;18:215–7.
Catalán V, Gómez-Ambrosi J, Rotellar F, et al. Validation of endogenous control genes in human adipose tissue: relevance to obesity and obesity-associated type 2 diabetes mellitus. Horm Metab Res. 2007;39:495–500.
Catalán V, Gómez-Ambrosi J, Rotellar F, et al. The obestatin receptor (GPR39) is expressed in human adipose tissue and is down-regulated in obesity-associated type 2 diabetes mellitus. Clin Endocrinol. 2007;66:598–601.
Wajchenberg BL. Subcutaneous and visceral adipose tissue: their relation to the metabolic syndrome. Endocr Rev. 2000;21:697–738.
Rodríguez A, Catalán V, Gómez-Ambrosi J, et al. Visceral and subcutaneous adiposity: are both potential therapeutic targets for tackling the metabolic syndrome? Curr Pharm Des. 2007;21:2169–75.
Kishida K, Shimomura I, Nishizawa H, et al. Enhancement of the aquaporin adipose gene expression by a peroxisome proliferator activated receptor γ. J Biol Chem. 2001;276:48572–9.
Acknowledgments
This work was supported by FIS PI030381, PI061458, and PI06/90288 from the Spanish Instituto de Salud Carlos III, Ministerio de Sanidad y Consumo and by the Department of Health of the Gobierno de Navarra (48/2003 and 20/2005) of Spain. The authors gratefully acknowledge the valuable collaboration of the surgery assistant nurses and the members of the Multidisciplinary Obesity Team. CIBER de Fisiopatología de la Obesidad y Nutrción (CIBEROBN) is an initiative of the Instituto de Salud Carlos III, Spain.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Catalán, V., Gómez-Ambrosi, J., Pastor, C. et al. Influence of Morbid Obesity and Insulin Resistance on Gene Expression Levels of AQP7 in Visceral Adipose Tissue and AQP9 in Liver. OBES SURG 18, 695–701 (2008). https://doi.org/10.1007/s11695-008-9453-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-008-9453-7