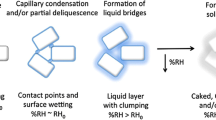
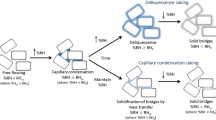
Abstract
This study was conducted to determine the changes in physico-chemical properties during storage of non-centrifugal sugar (NCS) made in the form of powder and solid blocks. The changes in reducing sugars (RS) and crystallinity were determined as a function of time and ambient conditions. Critical water activity (aw) values of both the forms of NCS were found such that product was stable when stored for 6 months at or below this water activity level. Sorption studies were also conducted for samples with different reducing sugar contents. The NCS samples with less RS, and that in powder form, showed less sorption and higher crystallinity, as compared to NCS with higher RS and made in the form of solid blocks. The reason for the occasional structural collapse of solid blocks was investigated, and the phenomenon was attributed to incomplete crystallization.








Similar content being viewed by others
Data availability
Included as supplementary material.
References
J.P.V.K. Rao, M. Das, S.K. Das, Effect of moisture content on glass transition and sticky point temperatures of sugarcane, palmyra-palm and date-palm jaggery granules. Int. J. Food Sci. Technol. 45(1), 94–104 (2010). https://doi.org/10.1111/j.1365-2621.2009.02108.x
A. Nath, D. Dutta, D.P. Kumar, J. Singh, Review on recent advances in value addition of jaggery based products. J. Food Process. Technol. 6(4), 4–7 (2015). https://doi.org/10.4172/2157-7110.1000440
W.R. Jaffé, Nutritional and functional components of non centrifugal cane sugar: a compilation of the data from the analytical literature. J. Food Compos. Anal. 43, 194–202 (2015). https://doi.org/10.1016/j.jfca.2015.06.007
J.P.V.K. Rao, M. Das, S.K. Das, Jaggery—a traditional indian sweetener. Indian J. Tradit. Knowl. 6(1), 95–102 (2007)
T.B. Deokate, S.N. Tilekar, Economics of production and marketing of jaggery in Maharashtra. Int. J. Commer. Bus. Manag. 2(2), 68–72 (2010)
T. Deokate, D. Bandgar, B. Mali, Marketing and Export of Jaggery, 1st edn. (Scholars’ Press, Saarbrücken, 2009)
J. Singh, R.D. Singh, S.I. Anwar, S. Solomon, Alternative sweeteners production from sugarcane in India: lump sugar (jaggery). Sugar Tech 13(4), 366–371 (2011). https://doi.org/10.1007/s12355-011-0110-4
V.K. Verma, M. Narain, Moisture absorption isotherms of jaggery. J. Stored Prod. Res. 26(2), 61–66 (1990). https://doi.org/10.1016/0022-474X(90)90001-9
P. Verma, N. Shah, S.M. Mahajani, Drying characteristics of non-centrifugal sugar. Dry. Technol. (2019). https://doi.org/10.1080/07373937.2019.1684318
P. Verma, N. Shah, S.M. Mahajani, Effects of acid treatment in jaggery making. Food Chem. 299(January), 125094 (2019). https://doi.org/10.1016/j.foodchem.2019.125094
P. Verma, N. Shah, S.M. Mahajani, Effect of sodium hydrosulphite treatment on the quality of non-centrifugal sugar: jaggery. Food Chem. 299, 125043 (2019). https://doi.org/10.1016/j.foodchem.2019.125043
Y.H. Roos, M. Karel, Applying state diagrams to food processing and development. Food Technol. 45(12), 66–107 (1991)
H.M.A. Nayaka, U.V. Sathisha, M.P. Manohar, K.B. Chandrashekar, S.M. Dharmesh, Cytoprotective and antioxidant activity studies of jaggery sugar. Food Chem. 115(1), 113–118 (2009). https://doi.org/10.1016/j.foodchem.2008.11.067
L. Seguí, L. Calabuig-Jiménez, N. Betoret, P. Fito, Physicochemical and antioxidant properties of non-refined sugarcane alternatives to white sugar. Int. J. Food Sci. Technol. 50(12), 2579–2588 (2015). https://doi.org/10.1111/ijfs.12926
D.H. Flórez-Martínez, C.A. Contreras-Pedraza, J. Rodríguez, A systematic analysis of non-centrifugal sugar cane processing: research and new trends. Trends Food Sci. Technol. 107, 415–428 (2021). https://doi.org/10.1016/j.tifs.2020.11.011. ISSN 0924-2244
P. Verma, N. Shah, S.M. Mahajani, Why jaggery powder is more stable than solid jaggery blocks. LWT 110(April), 299–306 (2019). https://doi.org/10.1016/j.lwt.2019.04.093
G.L. Miller, Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 31(3), 426–428 (1959). https://doi.org/10.1021/ac60147a030
L. Greenspan, Humidity fixed points of binary saturated aqueous solutions. J. Res. Natl. Bur. Stand. A 81A(1), 89 (1977). https://doi.org/10.6028/jres.081A.011
S. Singh, A. Dubey, L. Tiwari, A.K. Verma, Microbial profile of stored jaggery: a traditional Indian sweetener. Sugar Tech 11(2), 213–216 (2009). https://doi.org/10.1007/s12355-009-0034-4
R. Ergun, R. Lietha, R.W. Hartel, Moisture and shelf life in sugar confections. Crit. Rev. Food Sci. Nutr. 50(2), 162–192 (2010). https://doi.org/10.1080/10408390802248833
H.O. Hamad, H.M. Alma, H.M. Ismael, A. Goceri, The effect of some sugars on the growth of Aspergillus Niger. KSU J. Nat. Sci. 17(4), 7–11 (2014)
C. Molina-Ramírez, M. Castro, M. Osorio, M. Torres-Taborda, B. Gómez, R. Zuluaga, C. Gómez, P. Gañán, O. Rojas, C. Castro, Effect of different carbon sources on bacterial nanocellulose production and structure using the low PH resistant strain Komagataeibacter medellinensis. Materials (Basel) 10(6), 639 (2017). https://doi.org/10.3390/ma10060639
T.P. Labuza, Effect of water activity on reaction kinetics of food deterioration. Food Technol. (1980). https://doi.org/10.1016/j.ijfoodmicro.2005.10.002
M.S. Rahman, State diagram of foods: its potential use in food processing and product stability. Trends Food Sci. Technol. 17(3), 129–141 (2006). https://doi.org/10.1016/j.tifs.2005.09.009
M.A.J.S. van Boekel, Moisture content and water activity relations in honey: a Bayesian multilevel meta-analysis. J. Food Compos. Anal. 123, 105595 (2023). https://doi.org/10.1016/j.jfca.2023.105595. ISSN 0889-1575
L.R. Beuchat, Influence of water activity on growth, metabolic activities and survival of yeasts and molds. J. Food Prot. 46(2), 135–141 (1983). https://doi.org/10.4315/0362-028X-46.2.135
K. Kouassi, Y.H. Roos, Glass transition and water effects on sucrose inversion by invertase in a lactose-sucrose system. J. Agric. Food Chem. 48(6), 2461–2466 (2000). https://doi.org/10.1021/jf9902999
K. Kouassi, Y.H. Roos, Glass transition and water effects on sucrose inversion in noncrystalline carbohydrate food systems. Food Res. Int. 34(10), 895–901 (2001). https://doi.org/10.1016/S0963-9969(01)00114-4
Y.H. Roos, S. Drusch, Phase Transitions in Foods, 2nd edn. (Elsevier, Killington, 2016). https://doi.org/10.1016/B978-0-12-408086-7.00003-6
V. Guillard, C. Bourlieu, N. Gontard, Relationship between multiscale food structure and moisture transfer properties, in Food Structure and Moisture Transfer, vol. 5 (Springer New York, New York, 2013), pp. 35–44. https://doi.org/10.1007/978-1-4614-6342-9_3
E.J. Quirijns, A.J. van Boxtel, W.K. van Loon, G. van Straten, Sorption isotherms, GAB parameters and isosteric heat of sorption. J. Sci. Food Agric. 85(11), 1805–1814 (2005). https://doi.org/10.1002/jsfa.2140
Z.B. Maroulis, E. Tsami, D.M. Kouris, G.D. Saravacos, Application of the GAB model to the moisture sorption isotherms for dried fruits. J. Food Eng. 7, 63–78 (1988)
A.K. Shrestha, T. Howes, B.P. Adhikari, B.R. Bhandari, Water sorption and glass transition properties of spray dried lactose hydrolysed skim milk powder. LWT 40(9), 1593–1600 (2007). https://doi.org/10.1016/j.lwt.2006.11.003
S.J. Schmidt, M.A. Ruiz-Cabrera, C. Rivera-Bautista, A. Grajales-Lagunes, R. González-García, State diagrams for mixtures of low molecular weight carbohydrates. J. Food Eng. 171, 185–193 (2016). https://doi.org/10.1016/j.jfoodeng.2015.10.038
Y.H. Roos, Melting and glass transitions of low molecular weight carbohydrates. Carbohydr. Res. 238, 39–48 (1993)
A. Simperler, A. Kornherr, R. Chopra, P. Bonnet, W. Jones, W.D.S. Motherwell, G. Zifferer, Glass transition temperature of glucose, sucrose, and trehalose: an experimental and in silico study. J. Phys. Chem. B 110(39), 19678–19684 (2006). https://doi.org/10.1021/jp063134t
P. Verma, N. Shah, S.M. Mahajani, A novel technique to characterize and quantify crystalline and amorphous matter in complex sugar mixtures. Food Anal. Methods 13, 2087–2101 (2020). https://doi.org/10.1007/s12161-020-01789-1
P. Verma, N. Shah, S.M. Mahajani, Insights into the crystallization phenomenon in the production of non-centrifugal sugar. J. Food Eng. 290, 110259 (2020). https://doi.org/10.1016/j.jfoodeng.2020.110259. ISSN 0260-8774
B. Bhandari, R. Hartel, Co-crystallization of sucrose at high concentration in the presence of glucose and fructose. J. Food Sci. 67(5), 1797–1802 (2002). https://doi.org/10.1111/j.1365-2621.2002.tb08725.x
Y. Lu, L.C. Thomas, J.P. Jerrell, K.R. Cadwallader, S.J. Schmidt, Investigating the thermal decomposition differences between beet and cane sucrose sources. J. Food Meas. Charact. (2017). https://doi.org/10.1007/s11694-017-9544-z
M. Hurtta, I. Pitkänen, J. Knuutinen, Melting behaviour of d-sucrose, d-glucose and d-fructose. Carbohydr. Res. 339(13), 2267–2273 (2004). https://doi.org/10.1016/j.carres.2004.06.022
Y.H. Roos, F. Franks, M. Karel, T.P. Labuza, H. Levine, M. Mathlouthi, D. Reid, E. Shalaev, L. Slade, Comment on the melting and decomposition of sugars. J. Agric. Food Chem. 60(41), 10359–10362 (2012). https://doi.org/10.1021/jf3002526
F. Fan, Y.H. Roos, X-ray diffraction analysis of lactose crystallization in freeze-dried lactose-whey protein systems. Food Res. Int. 67, 1–11 (2015). https://doi.org/10.1016/j.foodres.2014.10.023
M.K. Haque, Y.H. Roos, Crystallization and X-ray diffraction of crystals formed in water-plasticized amorphous spray-dried and freeze-dried lactose/protein mixtures. J. Food Sci. 70(5), E359–E366 (2005). https://doi.org/10.1111/j.1365-2621.2005.tb09977.x
P. Verma, N. Shah, S.M. Mahajani, Scientific understanding of production of non-centrifugal sugar. J. Sugar Tech 23, 1393–1412 (2021). https://doi.org/10.1007/s12355-021-01006-1
T. Koji, M. Shinta, O. Takashi, I. Hiroyuki, I. Naoyuki, I. Koreyoshi, Water sorption and glass-to-rubber transition of amorphous sugar matrices, vacuum foam- and spray-dried from alcohols. J. Food Eng. 349, 111483 (2023). https://doi.org/10.1016/j.jfoodeng.2023.111483. ISSN 0260-8774
Acknowledgements
The authors thank Tata Centre for Technology and Design, IIT Bombay, for financial support. Authors thank Prof. Ramaswamy C. Anantheswaran, Professor at Food Science & Chair of the Cocoa, Chocolate and Confectionery Research Group, Penn State University, for his inputs. They also thank Mr. Vishwambhar Patil, Mr. Rohit Kumbhar, and the project staff involved in the field trial, for their support in conducting the experiments.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Ethical approval
No living being was part of this study. Ethical approval is not required for this study.
Consent to participate
Not applicable.
Consent to publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Verma, P., Shah, N.G. & Mahajani, S.M. Product instability studies of non-centrifugal sugar at different storage conditions. Food Measure 18, 1650–1663 (2024). https://doi.org/10.1007/s11694-023-02266-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11694-023-02266-z