Abstract
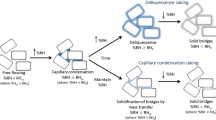
Typically, a crystalline powder is considered reasonably stable below it deliquescence point (RH0), however, caking has been reported for some materials below their RH0. The critical relative humidity (RH) values for caking and hydrate formation in alpha-anhydrous glucose (α-AG) and α-AG partitioned into three particle sizes were assessed using saturated salt slurries ranging from 0 to 84 % RH at 25 °C for 20 weeks. The degree of caking was determined by a five-point visual physical stability scale, from free flowing with minimal clumping (1) to fully caked (5), and X-ray powder diffraction was used to determine the composition of the samples. Caking was observed in α-AG during storage at 68 % RH at 25 °C and the severity of caking increased with increasing RH. Fine particle α-AG caked during storage at 64 % RH, whereas medium and large particle α-AG caked at 68 and 75 % RH, respectively, at 25 °C. Caking was observed in the absence of deliquescence, amorphous content, and hydrate formation; therefore, it is proposed that capillary condensation leads to caking in α-AG below its RH0. Capillary condensation caking occurs at a specific RH (termed RHcc) where direct condensation of moisture into confined spaces, such as particle contact points or surface defects, causes the formation of liquid bridges, which may solidify over time without changes in RH or temperature. To avoid caking, α-AG should be stored below its RHcc, which is highly dependent on particle size; and to avoid conversion to glucose monohydrate, α-AG, regardless of particle size, should be stored below 64 % RH at 25 °C.








Similar content being viewed by others
References
M. Peleg, J. Food Process. Eng. 1, 303–328 (1977)
M. Peleg, in Physical Properties Foods, ed. by M. Peleg, E.B. Bagley (AVI Publishing Company Inc, Westport, 1983), pp. 293–323
E.J. Griffith, Cake Formation in Particulate Systems (VCH Publishers Inc., New York, 1991), pp. 10–237
B.R. Bhandari, R.W. Hartel, in Encapsulated and Powdered Foods, ed. by C. Onwulata (Taylor and Francis, New York, 2005), pp. 261–292
P. Juliano, G.V. Barbosa-Cánovas, Annu. Rev. Food Sci. Technol. 1, 211–233 (2010)
L.E. Chuy, T.P. Labuza, J. Food Sci. 59(1), 43–46 (1994)
H. Rumpf, in Agglomeration, ed. by W. Knepper (Interscience Pubishers, New York, 1962), pp. 379–418
H. Burak, Chem. Ind. 21, 844–846 (1966)
M.P. Bhatt, D.S. Datar, Salt Res. Ind. 5(3/4), 57–67 (1968)
G.W. White, A.V. Bell, G.K. Berry, J. Food Technol. 2, 45–52 (1967)
B. Roge, M. Mathlouthi, Zuckerindustrie 125(5), 336–339 (2000)
M. Mathlouthi, B. Roge, Zuckerindustrie 126(11), 880–884 (2001)
M. Mathlouthi, B. Roge, Food Chem. 82, 61–71 (2003)
H. Hou, C.C. Sun, J. Pharm. Sci. 97(9), 4030–4039 (2008)
E. Teunou, J.J. Fitzpatrick, E.C. Synnott, J. Food Eng. 39, 31–37 (1999)
J.J. Fitzpatrick, S.A. Barringer, T. Iqbal, J. Food Eng. 61, 399–405 (2004)
D. Janjatović, M. Benković, S. Srečec, D. Ježek, I. Špoljarić, I. Bauman, Adv. Powder Technol. 23(5), 620–631 (2011)
S.J. Schmidt, Manuf. Confect. 92(1), 79–89 (2012)
J. Bronlund, T. Paterson, Int. Dairy J. 14, 247–254 (2004)
S.W. Billings, J.E. Bronlund, A.H.J. Paterson, J. Food Eng. 77, 887–895 (2006)
M. Wahl, U. Bröckel, L. Brendel, H.J. Feise, B. Weigl, M. Röck, J. Schwedes, Powder Technol. 188, 147–152 (2008)
R.M. Kirsch, R.A. Williams, U. Bröckel, R.B. Hammond, X. Jia, Ind. Eng. Chem. Res. 50(20), 11728–11733 (2011)
M. Wahl, R. Kirsch, U. Brockel, S. Trapp, M. Bottlinger, Chem. Eng. Technol. 29(6), 674–678 (2006)
P.J. Mulvihill, in Starch Hydrolysis Products, ed. by F.W. Schench, R.E. Hebeda (VCH Publishers Inc., New York, 1991), pp. 121–176
M. Rüegg, B. Blanc, Lebensmittel-Wissenschaft and Technologie 14(1), 1–6 (1981)
C.G. Peng, A.H.L. Chow, C.K. Chan, Aerosol Sci. Technol. 35(3), 753–758 (2001)
A.K. Salameh, L.J. Mauer, L.S. Taylor, J. Food Sci. 71(1), E10–E16 (2006)
S.K. Scholl, S.J. Schmidt, J. Food Sci. (2014). (accepted)
L.N. Bell, T.P. Labuza, Moisture Sorption Practical Aspects of Isotherm Measurement and Use, 2nd edn. (American Association of Cereal Chemists, St. Paul, 2000), pp. 12–45
S.K. Scholl, Investigation of glucose hydrate formation and loss: parameters, mechanisms, and physical stability [DPhil dissertation], (University of Illinois, Urbana-Champaign, 2014), p. 174
Decagon Devices Inc, AquaLab 4 Water Activity Meter Operator’s Man, 4.0th edn. (Decagon Devices Inc, Pullman, 2008), pp. 10–11
A.M. Stoklosa, R.A. Lipasek, L.S. Taylor, L.J. Mauer, Food Res. Int. 49, 783–791 (2012)
L. Greenspan, J. Res. Natl. Bur. Stand. Phys. Chem. 81A(1), 89–96 (1976)
J.F. Young, J. Appl. Chem. 17, 241–245 (1967)
Acknowledgments
The authors would like to thank Ingredion Incorporated (Westchester, IL) for providing alpha anhydrous glucose samples and Tate and Lyle (Hoffman Estates, IL) for carrying out the particle size analysis on α-AG samples. XRPD was carried out in part in the Frederick Seitz Materials Research Laboratory Central Research Facilities, University of Illinois at Urbana-Champaign, IL. Special thanks is given to Dr. Mauro Sardela, MRL Senior Research Scientist, for his expert technical assistance with x-ray diffraction procedures and analysis. Thanks is also given to Roman Kirsch from Dr. Ulrich Bröckel’s Laboratory at the Institute for Micro Process Engineering and Particle Technology at Umwelt-Campus Birkenfeld for feedback on the capillary condensation caking schematic.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Scholl, S.K., Schmidt, S.J. Determining the physical stability and water–solid interactions responsible for caking during storage of alpha-anhydrous glucose. Food Measure 8, 326–335 (2014). https://doi.org/10.1007/s11694-014-9193-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11694-014-9193-4