Abstract
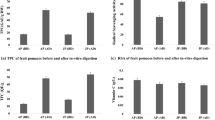
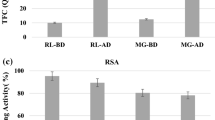
Loquat (Eriobotrya japonica Lindl.) is a perishable fruit with a huge potential for processing. In this study, the effects of food processing techniques and in vitro gastrointestinal digestion on the phenolics of loquat products were investigated. For this purpose, the results of processed loquat products including juice, nectar, leather, pulp, and jam were compared to the control loquat samples. Spectrophotometric and chromatographic methods were used to analyze the total phenolic (TPC) and total flavonoid contents (TFC), individual phenolic compounds (chlorogenic acid, p-coumaric acid, epicatechin) as well as total antioxidant capacity by DPPH and CUPRAC assays. The TPC and TFC of loquat products varied from 61.2±5.4 to 855.7±36.0 mg GAE/100 g and 37.2±1.6 to 979.5±65.3 mg CE/100 g, respectively. As in total phenolics, flavonoids, and antioxidants, the highest chlorogenic acid, p-coumaric acid, and epicatechin contents were obtained in loquat leather as 201.20±9.99 mg/100 g, 47.50±2.21 mg/100 g, and 46.87±0.28 mg/100 g, respectively. In addition to these, after in vitro gastrointestinal digestion, it was revealed that loquat leather had the highest TPC, TFC, and total antioxidant capacity values compared to other samples. Results have indicated that the phenolic content of fresh loquat fruits could be improved or protected, mainly by processing into leather and/or jam. Thus, the product scale for this fruit can be increased by obtaining foods with high nutritional value.
Similar content being viewed by others
Data Availability
Research data will be shared upon request.
References
S. Alfaro, A. Mutis, A. Quiroz, I. Seguel, E. Scheuermann, Effects of drying techniques on murtilla fruit polyphenols and antioxidant activity. J. Food Res. 3(5), 73 (2014). https://doi.org/10.5539/jfr.v3n5p73
M. Alminger, A.M. Aura, T. Bohn, C. Dufour, S.N. El, A. Gomes, S. Karakaya, M.C. Martínez-Cuesta, G.J. Mcdougall, T. Requena, C.N. Santos, In vitro models for studying secondary plant metabolite digestion and bioaccessibility. Compr. Rev. Food Sci. Food Saf. 13(4), 413–436 (2014). https://doi.org/10.1111/1541-4337.12081
R. Apak, K. Güçlü, M. Özyürek, S.E. Karademir, Novel total antioxidant capacity index for dietary polyphenols and vitamins C and E, using their cupric ion reducing capability in the presence of neocuproine: CUPRAC method. J. Agric. Food Chem. 52(26), 7970–7981 (2004). https://doi.org/10.1021/jf048741x
R. Apak, M. Özyürek, K. Güçlü, E. Çapanoğlu, Antioxidant activity/capacity measurement. 1. classification, physicochemical principles, mechanisms, and electron transfer (ET)-based assays. J. Agric. Food Chem. 64(5), 997–1027 (2016). https://doi.org/10.1021/acs.jafc.5b04739
F.J. Barba, C. Cortés, M.J. Esteve, A. Frígola, Study of antioxidant capacity and quality parameters in an orange juice–milk beverage after high-pressure processing treatment. Food Bioprocess Technol. 5(6), 2222–2232 (2012). https://doi.org/10.1007/s11947-011-0570-2
M.J. Bermudez-Soto, F.A. Tomas-Barberan, M.T. Garcia-Conesa, Stability of polyphenols in chokeberry (Aronia melanocarpa) subjected to in vitro gastric and pancreatic digestion. Food Chem. 102(3), 865–874 (2007). https://doi.org/10.1016/j.foodchem.2006.06.025
J. Bouayed, H. Deußer, L. Hoffmann, T. Bohn, Bioaccessible and dialysable polyphenols in selected apple varieties following in vitro digestion vs. their native patterns. Food Chem. 131(4), 1466–1472 (2012). https://doi.org/10.1016/j.foodchem.2011.10.030
J. Cai, T. Chen, Z. Zhang, B. Li, G. Qin, S. Tian, Metabolic dynamics during loquat fruit ripening and postharvest technologies. Front. Plant Sci. 10, 619 (2019). https://doi.org/10.3389/fpls.2019.00619
X. Cao, Y. Zhang, F. Zhang, Y. Wang, J. Yi, X. Liao, Effects of high hydrostatic pressure on enzymes, phenolic compounds, anthocyanins, polymeric color and color of strawberry pulps. J. Sci. Food. Agric. 91(5), 877–885 (2011). https://doi.org/10.1002/jsfa.4260
E. Capanoglu, J. Beekwilder, D. Boyacioglu, R. Hall, De R. Vos, Changes in antioxidant and metabolite profiles during production of tomato paste. J. Agric. Food Chem. 56(3), 964–973 (2008). https://doi.org/10.1021/jf072990e
Y. Chen, A. Martynenko, Combination of hydrothermodynamic (HTD) processing and different drying methods for natural blueberry leather. LWT-Food Sci. Technol. 87, 470–477 (2018). https://doi.org/10.1016/j.lwt.2017.09.030
B.P. Costa, M. Ikeda, de A.M. Melo, F.E.S.B. Alves, D. Carpine, R.H. Ribani, Eriobotrya japonica fruits and its by-products: a promising fruit with bioactive profile and trends in the food application–A bibliometric review. Food Biosci. 50, 102099 (2022). https://doi.org/10.1016/j.fbio.2022.102099
P.N. Curi, P.V. Nogueira, de A.B. Almeida, C.S. Carvalho, R. Pio, M. Pasqual, De V.R. Souza, Processing potential of jellies from subtropical loquat cultivars. Food Sci. Technol. (Brazil). 37(1), 70–75 (2017). https://doi.org/10.1590/1678-457X.07216
Del R. Pino-García, M.L. González-SanJosé, M.D. Rivero-Pérez, J. García-Lomillo, P. Muñiz, Total antioxidant capacity of new natural powdered seasonings after gastrointestinal and colonic digestion. Food Chem. 211, 707–714 (2016). https://doi.org/10.1016/j.foodchem.2016.05.127
I. Desseva, D. Mihaylova, Influence of in vitro gastrointestinal digestion on phytochemicals in pomegranate juice. Food Sci. Technol. 40, 211–216 (2020). https://doi.org/10.1590/fst.07219
I. Desseva, M. Stoyanova, N. Petkova, D. Mihaylova, Red beetroot juice phytochemicals bioaccessibility: an in vitro approach. Pol. J. Food Nutr. Sci. 70(1), 45–53 (2020). https://doi.org/10.31883/pjfns/116590
V. Dewanto, X. Wu, K.K. Adom, R.H. Liu, Thermal processing enhances the nutritional value of tomatoes by increasing total antioxidant activity. J. Agric. Food Chem. 50(10), 3010–3014 (2002). https://doi.org/10.1021/jf0115589
A. Dhiman, R. Suhag, D. Thakur, V. Gupta, P.K. Prabhakar, Current status of loquat (Eriobotrya Japonica Lindl.): bioactive functions, preservation approaches, and processed products. Food Rev. Int. 38, 286–316 (2022). https://doi.org/10.1080/87559129.2020.1866007
C.-K. Ding, K. Chachin, Y. Ueda, Y. Imahori, C.Y. Wang, Metabolism of phenolic compounds during loquat fruit development. J. Agric. Food Chem. 49(6), 2883–2888 (2001). https://doi.org/10.1021/jf0101253
S. Ercisli, S. Gozlekci, M. Sengul, A. Hegedus, S. Tepe, Some physicochemical characteristics, bioactive content and antioxidant capacity of loquat (Eriobotrya japonica (Thunb.) Lindl.) fruits from Turkey. Sci. Hort. 148, 185–189 (2012). https://doi.org/10.1016/j.scienta.2012.10.001
M.F. Erkölencik, Determination of bioactive and aromatic properties of different loquat varieties. Master thesis, Namik Kemal University, Tekirdag, Turkey (2016).
A. Gil-Izquierdo, M.I. Gil, F. Ferreres, F.A. Tomás-Barberán, In vitro availability of flavonoids and other phenolics in orange juice. J. Agric. Food Chem. 49(2), 1035–1041 (2001). https://doi.org/10.1021/jf0000528
V. Goulas, I.S. Minas, P.M. Kourdoulas, A.R. Vicente, G.A. Manganaris, Phytochemical content, antioxidants and cell wall metabolism of two loquat (Eriobotrya japonica) cultivars under different storage regimes. Food Chem. 155, 227–234 (2014). https://doi.org/10.1016/j.foodchem.2014.01.054
B. Guldiken, G. Toydemir, K.N. Memis, S. Okur, D. Boyacioglu, E. Capanoglu, Home-processed red beetroot (Beta vulgaris L.) products: changes in antioxidant properties and bioaccessibility. Int. J. Mol. Sci. 17(6), 858 (2016). https://doi.org/10.3390/ijms17060858
Z. Haida, M. Hakiman, A comprehensive review on the determination of enzymatic assay and nonenzymatic antioxidant activities. Food Sci. Nutr. 7(5), 1555–1563 (2019). https://doi.org/10.1002/fsn3.1012
M. He, J. Zeng, L. Zhai, Y. Liu, H. Wu, R. Zhang, Z. Li, E. Xia, Effect of in vitro simulated gastrointestinal digestion on polyphenol and polysaccharide content and their biological activities among 22 fruit juices. Food Res. Int. 102, 156–162 (2017). https://doi.org/10.1016/j.foodres.2017.10.001
A. Helal, D. Tagliazucchi, E. Verzelloni, A. Conte, Bioaccessibility of polyphenols and cinnamaldehyde in cinnamon beverages subjected to in vitro gastro-pancreatic digestion. J. Funct. Foods 7(1), 506–516 (2014). https://doi.org/10.1016/j.jff.2014.01.005
W. Huang, X. Bi, X. Zhang, X. Liao, X. Hu, J. Wu, Comparative study of enzymes, phenolics, carotenoids and color of apricot nectars treated by high hydrostatic pressure and high temperature short time. Innov. Food Sci. Emerg. Technol. 18, 74–82 (2013). https://doi.org/10.1016/j.ifset.2013.01.001
A. Ibarz, A. Garvin, J. Costa, Rheological behavior of loquat (Eriobotrya japonica) juices. J. Texture Stud. 27, 175–184 (1996). https://doi.org/10.1177/016555158501100107
M. Minekus, M. Alminger, P. Alvito, S. Ballance, T. Bohn, C. Bourlieu, F. Carrière, R. Boutrou, M. Corredig, D. Dupont, C. Dufour, L. Egger, M. Golding, S. Karakaya, B. Kirkhus, S. Le Feunteun, U. Lesmes, A. Macierzanka, A. Mackie, … A. Brodkorb, A standardised static in vitro digestion method suitable for food – an international consensus. Food Funct. 5(6), 1113–1124 (2014). https://doi.org/10.1039/C3FO60702J
S. Kamiloglu, M. Demirci, S. Selen, G. Toydemir, D. Boyacioglu, E. Capanoglu, Home processing of tomatoes (Solanum lycopersicum): effects on in vitro bioaccessibility of total lycopene, phenolics, flavonoids, and antioxidant capacity. J. Sci. Food. Agric. 94(11), 2225–2233 (2014). https://doi.org/10.1002/jsfa.6546
D.O. Kim, S.W. Jeong, C.Y. Lee, Antioxidant capacity of phenolic phytochemicals from various cultivars of plums. Food Chem. 81(3), 321–326 (2003). https://doi.org/10.1016/S0308-8146(02)00423-5
X. Li, C. Xu, K. Chen, Nutritional and composition of fruit cultivars. In V. R. Preedy, M.S.J. Simmonds (Eds.), Nutritional Composition of Fruit Cultivars (Academic Press, London, 2016), pp. 371–394. https://doi.org/10.1016/B978-0-12-408117-8.00016-7
D.B. López-Lluch, M. Cano-Lamadrid, F. Hernández, A. Zimmer, K. Lech, A. Figiel, Ã.A. Carbonell-Barrachina, A. Wojdyło, Hydroxycinnamic acids and carotenoids of dried loquat fruit cv. ‘Algar’ affected by freeze-, convective-, vacuum-microwave- and combined-drying methods. Molecules 25(16), 3643 (2020). https://doi.org/10.3390/molecules25163643
R. Martínez-Las Heras, A. Pinazo, A. Heredia, A. Andrés, Evaluation studies of persimmon plant (Diospyros kaki) for physiological benefits and bioaccessibility of antioxidants by in vitro simulated gastrointestinal digestion. Food Chem. 214, 478–485 (2017). https://doi.org/10.1016/j.foodchem.2016.07.104
G.J. McDougall, P. Dobson, P. Smith, A. Blake, D. Stewart, Assessing potential bioavailability of raspberry anthocyanins using an in vitro digestion system. J. Agric. Food Chem. 53(15), 5896–5904 (2005). https://doi.org/10.1021/jf050131p
D. Mihaylova, I. Desseva, M. Stoyanova, N. Petkova, M. Terzyiska, A. Lante, Impact of in vitro gastrointestinal digestion on the bioaccessibility of phytochemical compounds from eight fruit juices. Molecules 26(4), 1187 (2021). https://doi.org/10.3390/molecules26041187
A. Mishra, A. Upadhyay, P. Jaiswal, N. Sharma, Effect of different drying method on the chemical and microstructural properties of Loquat slices. J. Food Process. Preserv. 45, e15105 (2021). https://doi.org/10.1111/jfpp.15105
P. Molyneux, The use of the stable free radical diphenylpicryl-hydrazyl (DPPH) for estimating antioxidant activity. Songklanakarin J. Sci. Technol. 26(2), 211–219 (2004). https://doi.org/10.1287/isre.6.2.144
N. Ortega, A. Macià, M.-P. Romero, J. Reguant, M.-J. Motilva, Matrix composition effect on the digestibility of carob flour phenols by an in-vitro digestion model. Food Chem. 124(1), 65–71 (2011). https://doi.org/10.1016/j.foodchem.2010.05.105
A.T. Öz, E. Kafkas, A. Bozdoğan, Combined effects of oxalic acid treatment and modified atmosphere packaging on postharvest quality of loquats during storage. Turkish J. Agric. Forestry 40(3), 433–440 (2016). https://doi.org/10.3906/tar-1509-12
M.J. Rodríguez-Roque, M.A. Rojas-Graü, P. Elez-Martínez, O. Martín-Belloso, In vitro bioaccessibility of health-related compounds as affected by the formulation of fruit juice-and milk-based beverages. Food Res. Int. 62, 771–778 (2014). https://doi.org/10.1016/j.foodres.2014.04.037
M.J. Rodríguez-Roque, de B. Ancos, C. Sánchez-Moreno, M.P. Cano, P. Elez-Martínez, O. Martín-Belloso, Impact of food matrix and processing on the in vitro bioaccessibility of vitamin C, phenolic compounds, and hydrophilic antioxidant activity from fruit juice-based beverages. J. Funct. Foods. 14, 33–43 (2015). https://doi.org/10.1016/j.jff.2015.01.020
N.A. Sagar, S. Pareek, R. Bhardwaj, N. Vyas, Bioactive compounds of loquat (Eriobotrya japonica (Thunb.) L.). In H.N. Murthy, V.A. Bapat (Eds.), Bioactive compounds in underutilized fruits and nuts (Springer, New York, 2020), pp. 1–21. https://doi.org/10.1007/978-3-030-06120-3_10-1
K.L. Santos, P.H. Machado de Sousa, R.M. Cavalcanti-Mata, M.E., B. De Vasconcelos, L, Mixed leather of açaí, banana, peanut, and guarana syrup: the effect of agar and gellan gum use on quality attributes. Int. J. Gastronomy Food Sci. 26, 100407 (2021). https://doi.org/10.1016/j.ijgfs.2021.100407
H.M.S. Shah, A.S. Khan, Z. Singh, S. Ayyub, Postharvest biology and technology of loquat (Eriobotrya japonica Lindl.). Foods 12, 1329 (2023). https://doi.org/10.3390/foods12061329
V.L. Singleton, J.A. Rossi, Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Viticult. 16(3), 144–158 (1965).
S. Suna, A. Özkan-Karabacak, Investigation of drying kinetics and physicochemical properties of mulberry leather (pestil) dried with different methods. J. Food Process. Preserv. 43(8), 1–9 (2019). https://doi.org/10.1111/jfpp.14051
H. Takahashi, H. Sunitani, Y. Inada, D. Mori, Y. Nakano, Potent aroma volatiles in fresh loquat and its canned product. Nippon Shokuhin Kagaku Kogaku Kaisji 47(4), 302–310 (2000). https://doi.org/10.3136/nskkk.47.302
I. Tontul, A. Topuz, Effects of different drying methods on the physicochemical properties of pomegranate leather (pestil). LWT-Food Sci. Technol. 80, 294–303 (2017). https://doi.org/10.1016/j.lwt.2017.02.035
A. Topuz, Determination of some physical, chemical properties of loquat cultivars (Eriobotrya japonica L.) and possibilities of their being processed into marmalade, nectar and canned fruit. Master Thesis. Akdeniz University. Antalya, Turkey (1998).
TurkPatent, Alanya loquat (2018), https://ci.turkpatent.gov.tr/cografi-isaretler/detay/38373 Accessed 16 November 2022.
P.C. Wootton-Beard, L. Ryan, A beetroot juice shot is a significant and convenient source of bioaccessible antioxidants. J. Funct. Foods 3(4), 329–334 (2011). https://doi.org/10.1016/j.jff.2011.05.007
H. Xu, J. Chen, Commercial quality, major bioactive compound content and antioxidant capacity of 12 cultivars of loquat (Eriobotrya japonica Lindl.) Fruits. J. Sci. Food. Agric. 91(6), 1057–1063 (2011). https://doi.org/10.1002/jsfa.4282
W. Zhang, X. Zhao, C. Sun, X. Li, K. Chen, Phenolic composition from different loquat (Eriobotrya japonica Lindl.) cultivars grown in China and their antioxidant properties. Molecules 20(1), 542–555 (2015). https://doi.org/10.3390/molecules20010542
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
11694_2023_2179_MOESM1_ESM.docx
Supplementary Fig. 1: HPLC chromatograms at 280 nm for (a) control (fresh), (b) juice, (c) nectar, (d) leather, (e) pulp and (f) jam samples before digestion (1: chlorogenic acid; 2: p-coumaric acid; 3: epicatechin). Fig. 2: HPLC chromatograms at 280 nm for (a) control (fresh), (b) juice, (c) nectar, (d) leather, (e) pulp and (f) jam samples after gastric digestion (1: chlorogenic acid; 2: p-coumaric acid; 3: epicatechin). Fig. 3: HPLC chromatograms at 280 nm for (a) control (fresh), (b) juice, (c) nectar, (d) leather, (e) pulp and (f) jam samples after intestinal digestion (1: chlorogenic acid; 2: p-coumaric acid; 3: epicatechin)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ozkan, G., Oner, M.E., Tas, D. et al. Effect of various food processing techniques on the retention of loquat phenolics and their antioxidant capacity during in vitro digestion. Food Measure 18, 428–436 (2024). https://doi.org/10.1007/s11694-023-02179-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11694-023-02179-x