Abstract
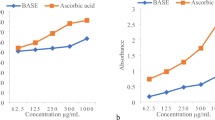
This study focused on the fenugreek seed powder extract (FSP-Ex) obtained using four selected solvents based on polarity differences (acetone, ethanol, hexane, and petroleum ether). The FSP-Ex properties were studied through pH, color, antioxidant, flavonoid and phenolic content. Phase transition by DSC and FTIR spectrum was performed to get more insight into FSP-Ex and antimicrobial activity against three pathogens. The maximum value of FSP-Ex was noticed with ethanol as compared to other solvents. The pH, color (L*a*b*), antioxidant, flavonoid, and phenolic content of ethanol FSP-Ex were 6.87 ± 0.03 (66.3,. 10.66 and 44.23), 67.87%, 97.17 ± 0.01 mg QE/g and 71.17 ± 0.01 mg GAE/g respectively. The analytical properties of FSP-Ex revealed its thermal behavior and the presence of functional groups in chemical structure by DSC and FTIR, respectively. The phase transition by DSC analysis and FTIR analysis generated peaks that revealed the thermal characterization and functional groups present in FSP-Ex. The antimicrobial activity of ethanolic extract of FSP revealed the most suited zone of inhibition of 3.27 mm against Bacillus subtilis at 100 mg/mL strength among other selected microbes and solvents. Hence, based on these findings, FSP-Ex could be considered a novel method to study the relationship between solvent polarity with FSP-Ex and its phytochemical, physicochemical, and analytical properties.





Similar content being viewed by others
Data availability
The datasets created for this study are available on the reasonable request of the corresponding authors.
References
K. Platel, K. Srinivasan, Nahrung 44, 42–46 (2000)
K.T. Roberts, J. Med. Food 14, 1485–1489 (2011)
P. Arya, P. Kumar, J. Food Biochem. 3(11), e14390 (2022)
D. Srivastava, J. Rajiv, M.M.N. Mahadevamma, J. Puranaik, P. Srinivas, Food Sci. Nutr. Sci. 3, 1473–1479 (2012)
H.S. Snehlata, D.R. Payal, Int. J. Curr. Pharm. Rev. Res. 2, 169–187 (2012)
D. Tewari, A. Jóźwik, M. Łysek-Gładysińska, W. Grzybek, W. Adamus-Białek, J. Bicki, N. Strzałkowska, A. Kamińska, O.K. Horbańczuk, A.G. Atanasov, Nutrients 12, 2522 (2020)
S.A. Wani, P. Kumar, J. Saudi Soc. Agric. Sci. 17, 97–106 (2018)
C. Zhou, Y. Qin, R. Chen, F. Gao, J. Zhang, F. Lu, Life Sci. 258, 1–9 (2020)
T.A. Dar, M. Uddin, J. Complement. Med. Altern. Healthc. 7, 1–3 (2018)
S. Chaudhary, P.S. Chaudhary, S.K. Chikara, M.C. Sharma, M. Iriti, Not. Bot. Horti Agrobot. 46, 22–31 (2018)
T. Hashidume, K. Sasaki, J. Hirata, M. Kato, Y. Yoshikawa, Y. Iwasaki, H. Arai, S. Miura, N. Miyoshi, J. Agric. Food Chem. 66, 9968–9975 (2018)
P. Aumsuwan, S.I. Khan, I.A. Khan, Z. Ali, B. Avula, L.A. Walker, Z. Shariat-Madar, W.G. Helferich, B.S. Katzenellenbogen, A.K. Dasmahapatra, Arch. Biochem. Biophys. 591, 98–110 (2016)
S.A. Wani, S. Bishnoi, P. Kumar, Food Meas. 10, 98–110 (2016)
T. Chen, S. Hu, H. Zhang, Q. Guan, X. Yang, X. Wang, Food Funct. 8, 659–669 (2017)
T. Wang, R.C. Choi, J. Li, C.W. Bi, W. Ran, X. Chen, T.T. Dong, K. Bi, K.W. Tsim, J. Ethnopharmacol. 139, 214–220 (2012)
W. Sun, M.H. Shahrajabian, H. Shen, M. Khoshkharam, Q.I. Cheng, Res. Crop Ecophysiol. 14, 52–65 (2019)
A. Binesh, S.N. Devaraj, H. Devaraj, Biochimie 148, 63–71 (2018)
K. Onoda, M. Kato, Y. Tsunematsu, F. Eto, M. Sato, Y. Yoshioka, T. Yoshida, K. Tamura, I. Yao, H. Dohra, K. Watanabe, N. Miyoshi, J. Agric. Food Chem. 71, 4292–4297 (2023)
P. Arya, P. Kumar, J. Food Biochem. 45(12), e14005 (2021)
Z. Chen, J. Xu, Y. Wu, S. Lei, H. Liu, Q. Meng, Z. Xia, Biochem. Biophys. Res. Commun. 503, 1181–1185 (2018)
M. Liu, L. Xu, L. Yin, Y. Qi, Y. Xu, X. Han, Y. Zhao, H. Sun, J. Yao, Y. Lin, K. Liu, J. Peng, Sci. Rep. 5, 1–11 (2015)
T. Xu, L. Zheng, L. Xu, L. Yin, Y. Qi, Y. Xu, X. Han, J. Peng, Arch. Toxicol. 88, 739–753 (2014)
X. Zhao, X. Cong, L. Zheng, L. Xu, L. Yin, J. Peng, Toxicol. Lett. 214, 69–80 (2012)
P. Arya, M. Munshi, P. Kumar, Food Chem. Adv. 2, 100170 (2023)
M.L. McKoy, P.G. Thomas, H. Asemota, F. Omoruyi, O. Simon, J. Med. Food 17, 1183–1188 (2014)
L. Sanchez-Sanchez, M.G. Hernandez-Linares, M.L. Escobar, H. Lopez-Munoz, E. Zenteno, M.A. Fernandez-Herrera, G. Guerrero-Luna, A. Carrasco-Carballo, J. Sandoval-Ramirez, Molecules 21, 1533 (2016)
E. Herrera-Pool, A.L. Ramos-Díaz, M.A. Lizardi-Jiménez, S. Pech-Cohuo, T. Ayora-Talavera, J.C. Cuevas-Bernardino, U. García-Cruz, N. Pacheco, Ultrason. Sonochem. 76, 105658 (2021)
A. Jouyban, S. Soltanpour, H.K. Chan, Int. J. Pharm. 269(2), 353–360 (2004)
H. Nawaz, M.A. Shad, N. Rehman, H. Andaleeb, N. Ullah, Braz. J. Pharm. Sci. 56, 5–11 (2019)
A.C. Akinmoladun, O.E. Falaiye, O.B. Ojo, A. Adeoti, Z.A. Amoo, M.T. Olaleye, Bull. Natl. Res. Cent. 46(1), 1–9 (2022)
O.K. Chun, A. Floegel, S.J. Chung, C.E. Chung, W.O. Song, S.I. Koo, J. Nutr. 140, 317–324 (2009)
L.F. Pedersen, P. Rojas-Tirado, E. Arvin, P.B. Pedersen, Aquac. Eng. 85, 9–14 (2019)
C.S. Dzah, Y. Duan, H. Zhang, C. Wen, J. Zhang, G. Chen, H. Ma, Food Biosci. 35, 1–9 (2020)
P.X. Chen, Y. Tang, M.F. Marcone, P.K. Pauls, B. Zhang, R. Liu, R. Tsao, Food Chem. 185, 298–308 (2015)
D. Su, R. Zhang, F. Hou, M. Zhang, J. Guo, F. Huang, Z. Wei, BMC Complement. Altern. Med. 14, 1–9 (2014)
X.J. Yang, B. Dang, M.T. Fan, Molecules 23, 1–20 (2018)
C.J. Chirayil, J. Abraham, R.K. Mishra, S.C. George, S. Thomas, Therm. Rheol. Meas. Tech. Nanomater. Character. (2017). https://doi.org/10.3390/molecules26206304
S. Wen, J. Liu, J. Deng, Minerals fluid inclusions, in Fluid Inclusion Effect in Flotation of Sulfide Minerals (Elsevier, 2019), pp.1–16
L.A.N. Al-Timimi, Asian Pac. J. Cancer Prev. 20(12), 3771–3776 (2019)
M. Munshi, P. Arya, P. Kumar, J. Oleo Sci. 1, 1349–1358 (2020)
P. Arya, P. Kumar, Ultrason. Sonochem. 74, 1–9 (2021)
N. Kapilraj, S. Keerthanan, M. Sithambaresan, J. Chem. 4, 1–6 (2019)
Y. Ohno, in IS&T NIP16 Conference, Vancouver, Canada (2000), pp.
W. Brand-Williams, M.E. Cuvelier, C. Berset, Lebens. Wissen. Technol. 28, 25–30 (1995)
J. Zinshen, T. Mengcheng, W. Jainming, Food Chem. 64, 555–559 (1999)
L. Singleton, R. Orthofer, R.M. Lamuela-Raventos, Methods Enzymol. 299, 52–178 (1999)
F.H.A. Fernandes, C.P. Santana, R.L. Santos, L.P. Correia, M.M. Conceição, R.O. Macêdo, A.C.D. Medeiros, J. Therm. Anal. Calorim. 113, 443–447 (2012)
V.S. Murali, V.N.M. Devi, P. Parvathy, M. Murugan, Mater. Today Proc. 45, 2166–2170 (2020)
O.J. Fakayode, T.T.I. Nkambule, Food Chem. 348, 129146 (2021)
B.C. Smith, Introduction to infrared spectroscopy, in Fundamentals of fourier transform infrared spectroscopy (CRC Press, Boca Raton, 1996), pp.1–18
R. Farahmandfar, R. Esmaeilzadeh Kenari, M. Asnaashari, D. Shahrampour, T. Bakhshandeh, Food Sci. Nutr. 7(2), 465–475 (2019)
P. Kuppusamy, M.M. Yusoff, N.R. Parine, N. Govindan, Saudi J. Biol. Sci. 22(3), 293–301 (2015)
A.K.G. Harish, K. Ram, B. Singh, M. Phulwaria, N. Shekhawat, Libyan Agr. Res. Cent. J. Int. 2, 150–154 (2011)
A. Barchan, M. Bakkali, A. Arakrak, R. Pagán, A. Laglaoui, Int. J. Curr. Microbiol. Appl. Sci. 3, 1–15 (2014)
R. Saad, F. Asmani, M. Saad, M. Hussain, J. Khan, M. Kaleemullah, N.B. Othman, A. Tofigh, E. Yusuf, Int. J. Pharmacogn. Phytochem. Res. 7, 166–174 (2015)
R. Abarca-Vargas, R.V. Guerrero, V.L. Petricevich, Clin. Exp. Pharmacol. 6, 1–2 (2016)
A. Mansouri, G. Embarek, E. Kokkalou, P. Kefalas, Food Chem. 89, 411–420 (2005)
L.L. Mensour, F.S. Menezes, G.G. Leitao, A.S. Reis, T.C. Dos Santos, C.S. Coube, Phytother. Res. 15, 127–130 (2011)
Fotie, Pharmacogn. Rev. 2, 6–19 (2008)
Q.D. Do, A.E. Angkawijaya, P.L. Tran-Nguyen, L.H. Huynh, F.E. Soetaredjo, S. Ismadji, Y. Ju, J. Food Drug Anal. 22, 296–302 (2014)
M.A. Johari, H.Y. Khong, Adv. Pharmacol. Sci. 10, 1–4 (2019)
I.A. Almusallam, I.A.M. Ahmed, E.E. Babiker, F.Y. Al Juhaimi, G.J. Fadimu, M.A. Osman, S.A. Al Maiman, K. Ghafoor, H.A.S. Alqah, LWT 140, 110816 (2021)
H. Nawaz, M. Aslam, S.T. Muntaha, Free Radic. Antioxid. 9, 5–11 (2019)
A.I. Dirar, D.H.M. Alsaadi, M. Wada, M.A. Mohamed, T. Watanabe, H.P. Devkota, S. Afr. J. Bot. 120, 261–267 (2019)
L. Jing, H. Ma, P. Fan, R. Gao, Z. Jia, BMC Complement. Altern. Med. 15, 1–12 (2015)
S. Aryal, M.K. Baniya, K. Danekhu, P. Kunwar, R. Gurung, N. Koirala, Plants 8, 1–12 (2019)
A.B. Shoib, A.M. Shahid, J. Taibah Univ. Sci. 9, 449–454 (2015)
M.A. Soobrattee, V.S. Neergheen, A. Luximon-Ramma, O.I. Aruoma, T. Bahorun, Mutat. Res. Fundam. Mol. Mech. Mutagen. 579, 200–213 (2005)
A. Wojdylo, J. Oszmianski, R. Czemerys, Food Chem. 105, 940–949 (2007)
S.M.T. Gracia, M. Heinonen, E.N. Frankel, J. Agric. Food Chem. 45, 3362–3367 (1997)
H. Noreen, N. Semmar, M. Farman, J.S.O. McCullagh, Asian Pac. J. Trop. Med. 10, 792–801 (2017)
D. Jain, S. Shrivastava, Int. J. Eng. Technol. Sci. Res. 4, 2394–3386 (2017)
L.F. Lobato-Silva, W. Paschoal Jr., G.S. Pinheiro, J.G. da Silva Filho, P.T.C. Freire, F.F. de Sousa, S.G.C. Moreiraa, CrystEngComm 21, 1–21 (2019)
L.I.L. Favaro, V.M. Balcão, L.K.H. Rocha, E.C. Silva, J.M. Oliveira Jr., M.M.D.C. Vila, M. Tubino, J. Braz. Chem. Soc. 29, 2072–2088 (2018)
H.A. Deshpande, S.R. Bhalsing, Physiol. Mol. Biol. Plants 20, 89–94 (2014)
P. Kalailingam, K. Bhuvansehwari, B. Kunthavai, T. Evera, K. Rajendran, J. Planar Chromatogr. 25, 566–570 (2012)
F. Zhang, B. Shen, W. Jiang, H. Yuan, H. Zhou, J. Nanopart. Res. 21, 1–11 (2019)
I. Elsayed, M. Mashaly, F. Eltaweel, M.A. Jackson, E.B. Hassan, Fuel 221, 407–416 (2018)
M.S. Mirhosseyni, F. Nemati, A. Elhampour, J. Iran. Chem. Soc. 14, 791–801 (2016)
J.G. Shao, X.C. Xie, Y.J. Xi, X.N. Liu, Y.X. Yang, Glass Phys. Chem. 39, 329–335 (2013)
F. Alemi-Tameh, J. Safaei-Ghomi, M. Mahmoudi-Hashemi, R. Teymuri, Res. Chem. Intermed. 42, 6391–6406 (2016)
Y.L. Che, Y. Xu, R.J. Wang, L. Chen, Anal. Bioanal. Chem. 409, 4709–4718 (2017)
H.T. Zhou, X.F. Yu, G.M. Zhou, Mol. Med. Rep. 15, 2823–2828 (2017)
S. Cong, Q. Tong, Q. Peng, T. Shen, X. Zhu, Y. Xu, S. Qi, Mol. Med. Rep. 22(6), 5392–5398 (2020)
S.D.C. Beristain-Bauza, P. Hernández-Carranza, T.S. Cid-Pérez, R. Ávila-Sosa, I.I. Ruiz-López, C.E. Ochoa-Velasco, Food Rev. Int. 35(5), 407–426 (2019)
J.O. Akullo, B. Kiage, D. Nakimbugwe, J. Kinyuru, Heliyon 8(9), e10457 (2022)
A. Borges, H. José, V. Homem, M. Simões, Antibiotics 9(2), 1–19 (2020)
Q.L. Ren, Q. Wang, X.Q. Zhang, M. Wang, H. Hu, J.J. Tang, X.L. Li, Chin. J. Integr. Med. 11655, 1–12 (2023)
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
PA conducting experiments, interpreting the results, writing original draft. PK concept formulation reviewing and editing the original draft, supervision of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known personal relationships and financial interests that could have appeared to influence the work reported in this research paper.
Ethical approval
This research does not include any animal or human participants for experiments.
Informed consent
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Arya, P., Kumar, P. Phytochemical, phase transition, FTIR, and antimicrobial characterization of defatted Trigonella foenum graecum seed extract as affected by solvent polarity. Food Measure 17, 5234–5246 (2023). https://doi.org/10.1007/s11694-023-02028-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11694-023-02028-x