Abstract
Saw palmetto seeds (SPS) contain essential phenolic compounds that provide antioxidant, antimicrobial, anti-inflammatory, and anti-diabetic benefits when added to food. Maximized/improved production of these valuable phenolic compounds is the main purpose of this study. Solid-state fermentation (SSF) is a promising processing technique that positively alters the levels of health-promoting compounds in plants and plant residues. Here, a central composite design matrix (16 runs) and response surface methodology were experimentally applied to investigate the best SSF conditions and their interactions for maximum production of phenolic compounds from SPS. A good correlation between actual and expected results was observed with higher multiple coefficients (R2 ~ 0.93–0.97) and strongly significant P values (< 0.0001) proving the accuracy of the statistical model/design. Under optimized SSF conditions, temperature 30 °C, moisture 10%, pH 7.0, and fermentation time 6 days, the total phenolic content and total antioxidant activity of SPS were maximized by 11-fold and 46–49 folds, respectively. According to HPLC analysis, the contents of all identifying polyphenols were 3.3–30.0 times greater in fermented SPS extract (FSPS) than in the unfermented SPS extract (UFSPS). The FSPS extract also contained four new/additional polyphenols (vanillic, p-coumaric, cinnamic, and quercetin). FSPS extract demonstrated much greater antibacterial and antifungal activities than UFSPS extract against various human pathogenic bacteria and fungi. Consequently, the FSPS-phenolic compounds can be exploited as a food supplement and an antimicrobial remedy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Phenolic compounds are secondary plant metabolites that protect plants against UV radiation and several diseases [1]. These compounds are naturally occurring in a variety of foods such as vegetables, cereals, fruits, and beverages [2]. Following well-balanced nutritional diets rich in phenolic compounds can prevent several diseases such as cancer, obesity, osteoporosis, neurodegenerative defects, and diabetes mellitus [3, 4]. Phenolic compounds as antioxidants scavenge harmful reactive oxygen and nitrogen species created during metabolic reactions in the human body. In addition, these compounds possessed potential bioactivities such as antimicrobial, anti-inflammatory, anti-snake, anti-aging, and anti-mutagenic activities [5,6,7]. The phenolic compounds also extend the shelf life, improve color stability, and delay the formation of undesirable tastes in food products. Therefore, the antioxidant-phenolic compounds become a key addition in the food industry to improve shelf-life, nutritional value, and health-related properties [8]. Phenolic compounds are one of the most important dietary sources in the world. By 2025, the global market for phenolic compounds needs is expected to be valued at $2.08 billion [9].
Huge amounts of plant residues/wastes including seeds, peels, brans, straw, and pomaces are produced per year. These residues are rich/low-cost sources of valuable antioxidant-phenolic compounds, where they have no value or utilization. The reuse of plant residues and extraction of phenolic compounds is considered the main target for nutritionists [10]. Many extraction methods of phenolic compounds from plant and plant materials have been applied. Most of the traditional extraction methods are not environmentally friendly. The use of organic solvents during phenolic compound extraction is not preferred due to the high expense of organic solvents and the risk of contamination [11, 12]. Eco-friendly extraction methods have been developed including ultrasounds, microwave, maceration, and high hydrostatic pressure [13]. However, after applying these methods, low recovery of phenolic compounds was obtained because most of them are conjugated with sugars, amino acids, or fatty acids and/or are bound to plant cell walls. Therefore, they cannot be fully extracted by these methods [14].
Solid-state fermentation (SSF) is a biologically safe method that could produce a variety of hydrolytic enzymes. These enzymes could easily extract/liberate the bound/conjugated phenolics [15]. Additionally, low-cost growth mediums without pretreatment and reduced wastewater production are the best characteristics of the SSF method. The growth-medium costs up to 40% of the total production cost, therefore, cheaply available plant materials can be employed as a low-cost carbon source for phenolic compounds production [16]. Furthermore, the SSF method showed high efficiency for phenolic compounds liberation from plant materials and reduced solvents consumption as well as enhanced the bioactivities of various plant materials [15, 17, 18]. However, in a few cases, additional supplements may be added to the plant materials/wastes due to these materials being insufficient for microorganisms. In other cases, wastes were subjected to chemical pretreatment to produce media easier for microbial uptake. SSF could overcome some difficulties with mass and heat transport, scaling up, estimating biomass, and recovery control by applying some statistical experimental designs [19]. This statistical approach could resolve these difficulties and has the potential to save time and reduce the cost of the final product [20].
Saw palmetto (Serenoa repens) is a type of dwarf palm tree belonging to the Arecaceae family, originating in North America and widely cultivated as ornamental palms in Egypt. Berries extract of the saw palmetto (SPE) is the most common dietary supplement used to treat benign prostatic hyperplasia in men. The SPE also showed many pharmacological effects as antiproliferative, anti-inflammatory, and adrenoceptor-inhibitory properties [21]. On the other hand, the saw palmetto seeds extract (SPS) contains a significant amount of valuable antioxidant phenolic compounds with antimicrobial, anti-inflammatory, and anti-diabetic properties [4, 6]. However, no information about the influence of the SSF on the release of the antioxidant-polyphenols from saw palmetto seeds and their health-related properties. From this standpoint, a statistical design was implicated in this study to optimize SSF-physiological conditions for maximizing the release of the antioxidant-polyphenols from saw palmetto seeds by Trichoderma reesei, as well as to investigate their health benefits and functional properties.
Materials and methods
Materials
The Egyptian saw palmetto seeds (SPS) were identified and supplied by the Giza Agriculture Research Center. The SPS was washed, dried overnight in the oven at 40 °C, and milled. Trichoderma reesei was provided by the Microbial Chemistry Department, National Research Centre (NRC) Giza, Egypt. Trichoderma strain was re-cultivated for 7 days at 28 °C on potato dextrose agar and kept at 4 °C for later use. Escherichia coli O157-H7 ATCC 51659 and Staphylococcus aureus ATCC 13565 were supplied from the Food Toxins and Contaminants Department, NRC, Giza, Egypt. Fusarium proliferatum MPVP 328, Aspergillus parasiticus SSWT 2999 and Penicillium verrucosum BFE500 were supplied from Applied Mycology Department, Cranfield University, UK.
SSF-optimization conditions, statistical design, and phenolic compounds extraction
The SPS powder was disinfected with 70% ethanol followed by air-drying. In a flask, two grams of sterile SPS powder were inoculated with fresh Trichoderma reesei spores at 8.0 × 108 CFU/ml concentration. Herein, RSM (response surface methodology) and SCCD (statistical central composite design) were applied for the optimization of SSF conditions. Temperature, moisture content, incubation time, and pH, as significant SSF conditions for microbial growth and metabolite production were selected to optimize SPS fermentation. Based on single-parameter preliminary experiments, the ranges of SSF-conditions were selected at pH (6.5–7.0), temperature (30–35), incubation/fermentation time (6.0–7.0 days), and moisture content (5.0–10.0%) as central points for response surface methodology-optimization design and16 runs/experiments were conducted. After the optimized SSF process, the fermented SPS (FSPS) and unfermented SPS (UFSPS) were distributed in 80 percent methanol at 1:15 (w/v) and incubated overnight at 28 °C with shaking at 120 rpm. Whatman-1 filter papers were used to filter the obtained extracts.
Assessment of total phenolic content (TPC)
The TPC was assessed using the Velioglu et al. [22] method. For 5 min, a reaction mixture of 100 µl methanol extract, 100 µl Folin Ciocalteu reagent, and 800 µl distilled water, was incubated. Then, 500 µl of sodium carbonate solution (20%) was added to the reaction mixture, and incubation took place for 30 min. The absorbance was read at 750 nm. The TPC was measured in mg of gallic acid equivalent (GAE) per gram of seeds.
HPLC-analysis
HPLC technique was utilized to examine the polyphenolic compounds by Agilent Technologies1100 series liquid chromatography [23]. The XDB-C18 column (150 × 4.6 m) identified and separated the phenolic compounds. Acetonitrile and 2% acetic acid were used to elute the column at a 1 ml/min rate. Peaks were detected at 280, 320, and 360 nm and verified by phenolic compound standards and retention times.
Antioxidant properties
In the DPPH assay: 20 µl extract, 100 µl DPPH reagent (10 mM), and 880 µl methanol were mixed and incubated in the dark for 20 min, followed by reading the absorbance at 517 nm [24]. DPPH free-radical scavenging activity % = (Control absorbance − sample absorbance/control absorbance) × 100.
In the ABTS assay: 5–10 µl extract and 1.0 ml ABTS reagent were incubated for 1 min followed by reading the absorbance at 734 nm [25]. ABTS free-radical scavenging activity % was measured as mentioned above.
Antimicrobial properties
For antibacterial activity, a well diffusion strategy and Mueller–Hinton agar plates were utilized to examine the potency of the FSPS extract in comparison with the UFSPS extract [26]. The bacterium suspension (100 µl) was spread over the agar plate at a concentration of 108 CFU/ml. Wells were punched in the agar plates and filled with the FSPS and UFSPS extracts, each at a concentration of 50 µg GAE. The plates were then incubated for 18 h at 37 °C. For antifungal activity, each pathogenic fungal strain (100 µl) was spread over the potato dextrose agar at a 108 CFU/ml concentration. Wells were filled with the FSPS and UFSPS extracts, each at a concentration of 50 µg GAE. The plates were then incubated for 24 h at 26 ± 1 °C. Either antibacterial or antifungal activity was determined by measuring the diameters of inhibitory zones.
To determine the minimum inhibition concentration (MIC), various concentrations of FSPS and UFSPS extracts were added to contaminant agar plates and maintained for 18 ± 1 h at 37 ± 1 °C or 24 h at 26 ± 1 °C for antibacterial and antifungal activities, respectively. MIC is the minimum concentration at which bacteria/fungi cannot grow [27].
Statistical analysis
The SSF-physiological conditions were optimized using RSM and SCCD (Factorial statistical design expert V11 software, Stat-Ease Inc., US). A matrix of 16 runs was conducted, and the statistical results, model design, and equation were all validated using ANOVA with a P value of less than 0.05. The remaining data were examined with a one-way ANOVA followed by a Tukey post-test (GraphPad Prism 5 software). All values were presented as means ± SD (n = 4).
Results and discussion
SSF-optimization via statistical experimental design
During SSF, the production of phenolic compounds is heavily influenced by microbial growth conditions and fermentation media. The SSF-condition optimization is a difficult task because these independent conditions interact with one another. Therefore, the statistical approach is a key tool for overcoming these challenges, as it allows for more time and lowers costs in the production of phenolic compounds. Here, RSM and SCCD were applied to improve/adjust the SSF conditions (pH, temperature, fermentation time, and moisture content) for maximum phenolic compounds production from SPS. Table 1 lists the central points of the SSF conditions and compares the actual and expected SPS-phenolic contents resulting from 16 different experimental runs. To validate the actual and expected phenolic content values, statistical models and ANOVA variance analysis were conducted. According to this analysis, the 2FI model was proposed for this response (SPS-phenolic content) with strongly significant F-values and P values as listed in Table 2. In addition, the analyzed data showed that the adjusted R2 (0.9619) is substantially compatible with the expected R2 (0.9310) as well as the ‘Adeq accuracy’ signal-to-noise ratio is higher than four, which is ideal (Table 3). Furthermore, the actual and expected SPS-phenolic levels were also extremely near the fitting line, proving the validation/effectiveness of the suggested model (Fig. 1).
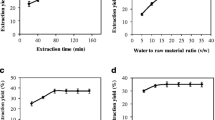
The impact of the examined SSF conditions and their mutual interactions on the production of SPS-phenolic compounds is depicted in Fig. 2a–d and Table 2. The results revealed that pH (B), and moisture content (D) have a significant influence on the SPS-phenolic level. However, at high levels, temperature (C) and incubation time (A) showed a negative/detrimental impact on SPS-phenolic compound production. In addition, there were significant mutual interactions between the A–C, A–D, B–D, and C–D parameters of SSF. Furthermore, the detrimental impact of A and C on the SPS-phenolic release/production was transformed to positive by interaction with C or D, and A or D, respectively.
Final Equation for maximum SPS-phenolic content production = + 26.50 – 0.3250A + 0.6083B – 1.68C + 1.14D– 0.0833AB + 0.2083AC – 0.2188AD – 0.0833BC + 0.0781BD-0.2188CD.
The coded equation is useful for identifying the relative impact of the SSF-conditions by comparing the condition coefficients. Figure 3 demonstrates the optimal SSF-conditions and the maximum predicted phenolic content using numerical maximization and desirability function. The maximum predicted phenolic level (31.13 mg GAE/g) was very near to the actual level (33.74 mg GAE/g) with a desirability function of 0.758 and a relative error percent of 7.7% as listed in Table 4. Overall, the efficiency of the independent SSF-conditions and their interactions, as well as the maximum SPS-optimized phenolic levels were estimated using the minimum experimental runs and a statistical model. These findings refer to the adequacy and precision of the applied statistical design. The RSM and CCD were also successfully applied in the optimization of the SSF-conditions to produce phenolic compounds from garden cress seeds and chia seeds [15, 18], flavonoid compounds from dandelion [28], and many microbial enzymes [15, 18, 29, 30].
The ideal SSF-conditions for maximum release of SPS-phenolic compounds were determined at a temperature of 30 °C, 10% moisture, pH 7.0, and 6-day fermentation duration (Table 4). The physiological conditions selected have an important influence on the effectiveness of the SSF process. Extensive fermentation time resulted in a reduction in phenolic content. The phenolic compounds may be consumed or degraded during fungal metabolism [31]. Low moisture limits microbial nutrition, proliferation, and growth, while higher moisture reduces the structure and porosity of medium particles. Changes in pH also reduced microbial enzyme release and antioxidant phenolics’ ability to contribute electrons. In addition, the higher temperature reduces microbial growth, and enzyme activities as well as increases molecular mobility, and hastens the breakdown of phenolic compounds. The most suitable SSF conditions for producing antioxidant polyphenolics from maize, bush tea, ginger leaves, garden cress seeds, and chia seeds were previously identified at relatively low moisture contents of 20 to 30%, low temperatures of 28 to 30 °C, pH of 6.0 to 7.0 and incubation period of 5 to 7 days [15, 17, 18, 32, 33]. On the other hand, the ideal SSF conditions for producing/releasing flavonoids from dandelions were optimized at a temperature of 35 °C, a moisture content of 52%, and an incubation period of 52 h [28].
Upgraded TPC of SPS
Under these optimal SSF conditions, the TPC is enhanced by 11 folds from 3.20 mg GAE/g for UFSPS extract to 33.74 mg GAE/g for FSPS extract as seen in Table 5. Likewise, under SSF by fungi, the TPC of various plants jumped several times. For instance, the TPC of fermented chia seeds, garden cress seeds, curry leaves, ginger leaves, and rice bran increased by ~23, 9, 5–6, 6.3, and 3-folds, respectively, compared to their unfermented/native forms [15, 17, 18, 34, 35]. This increase in TPC was due mainly to the liberation of free phenolic compounds from SPS as well as the production of new phenolic compounds. SPS previously recorded TPC ranging from 0.25 to 7.75 mg GAE/g when extracted by different solvents [6]. Furthermore, the TPC of SP-berries and SP-leaves were 14.3 and 6.3 mg GAE/g, respectively [36], demonstrating that the SSF process generated the highest TPC from SP-plant.
Impact of SSF on the SPS-phenolics composition
Table 6 displays the composition and concentration of phenolic compounds in the FSPS and UFSPS extracts using the HPLC method. Seven phenolics were found in the UFSPS extract, with phenolic contents ranging from 0.01 to 1.20 mg/g. While eleven phenolics were demonstrated in FSPS extract, with phenolic contents ranging from 0.1 to 4.0 mg/g. Four additional/new phenolic compounds (vanillic, p-coumaric, cinnamic, and quercetin) were discovered in the FSPS extract. The contents of all determining phenolics in the FSPS were 3.3–30.0 times higher than in the UFSPS extract. Protocatechuic was the major phenolic compound found in both SPS extracts, with a higher concentration in the FSPS extract (4.0 mg/g) than in the UFSPS extract (1.2 mg/g). Protocatechuic acid was also reported as the major phenolic compound in the SPS acetone extract (1.41 mg/g) [6]. Interestingly, FSPS extract contained 30 times more syringic acid (0.9 mg/g) than UFSPS extract (0.03 mg/g). Generally, the majority of phenolic compounds were structurally attached to the cell wall matrices of the plants. Therefore, this increase in polyphenol contents as well as producing new polyphenols in the FSPS extract under SSF is a result of enhanced activities of some fungal enzymes. The fungal enzymes decompose plant cell walls, transform many bound/insoluble phenolics into soluble and free phenolics, or generate new phenolics [15, 37]. In a similar trend, in fermented chia seeds, p-hydroxybenzoic, gallic, chlorogenic, cinnamic, and chrysin increased by 210, 36.4, 35.4, 23.3, and 20 folds, respectively, compared to unfermented chia seeds [15]. In fermented rice bran, coumaric acid, sinapic acid, and ferulic acid elevated by 20, 24, and 63 folds, respectively, when compared to native rice bran [35]. Ferulic acid increased by 3.82-fold in fermented whole-grain wheat [38]. Some new phenolics were also produced in many plants under SSF such as apigenin and kaempferol that appeared in fermented chia seeds [15], caffeic acid in fermented rice bran [35], apigenin in fermented garden cress seeds [18], and rutin and chlorogenic in fermented chickpeas [39]. Notably, most polyphenols identified in the FSPS have a wide range of therapeutic benefits like anti-aging, anti-inflammatory, antimicrobial, anti-snake, anticancer, antidiabetic, antiendotoxic, antioxidant, and hepatoprotective activities [3,4,5,6, 18, 40, 41]. According to these findings, FSPS-polyphenols can be employed as part of a dietary program to provide nutrition and health-promoting benefits.
Impact of SSF on the SPS-antioxidant activity
Under the SSF ideal conditions, the antioxidant activity of FSPS extract in comparison with UFSPS extract was evaluated. As seen in Table 5, the DPPH and ABTS IC50 results of UFSPS (12.5 and 3.5 µg GAE/ml, respectively) were markedly higher than for FSPS extract (2.7 and 0.8 µg GAE/ml, respectively). Much higher IC50 values were reported for SPS, SP-berries, and SP-leaves extracts (10.0, 294, and 1275 µg GAE/ml, respectively, for scavenging DPPH radicals) and (2.0, 234, and 724 µg GAE/ml, respectively, for scavenging ABTS radicals) [6, 36]. Strong antioxidant activity is evidenced by a lower IC50 value. In addition, by using the IC50 values of DPPH and ABTS, the total antioxidant activity (TAA) in FSPS extract (12,496 and 42,175, respectively) was considerably higher than in UFSPS extract (256 and 914, respectively) by 49 and 46 times, respectively. Interestingly, the FSPS-antioxidant activity was strongly correlated to its phenolic concentration. Therefore, this enhancement in the FSPS-antioxidant activity is due mainly to a considerable increase in the phenolic content. Additionally, most of the phenolic compounds determined in the FSPS are potent antioxidants. Most plant polyphenols are found attached to sugar polymers, resulting in a decrease in their antioxidant properties. Certain enzymes synthesized during SSF liberate/release the conjugated phenolic compounds, improving their antioxidant properties. Some enzymes can also produce new phenolic compounds and aglycones [15]. Aglycones are employed in a range of foods and beverages because of their high antioxidant activity [42]. Many previous studies have shown that SSF has a positive impact on the antioxidant capacity of some plants/plant residues such as wheat grain [43], wheat bran [44], rice [45], rice bran [35], black bean, soybean [46], ginger leaves [17], curry leaves [34], garden cress seeds [18], and chia seeds [15].
Impact of SSF on the SPS-antibacterial potency
The antibacterial potency of UFSPS and FSPS phenolic extracts at 50 µg GAE/ml was examined against E. coli (gram-negative) and S. aureus as (gram-positive) human pathogenic bacterial strains, respectively. Both E. coli and S. aureus are resistant to a variety of antibiotics and phytochemicals [6]. In Table7, the FSPS displayed significantly higher (P < 0.01) antibacterial potency against the studied bacteria (20.0–25.0 mm inhibition diameters) compared to UFSPS (10.0–14.0 mm) and Amoxicillin (15.0–16.0 mm). The FSPS exhibited markedly decreased (P < 0.01) MIC values against the studied bacteria, ranging from 0.2–0.3 mg/ml over the UFSPS (2.3–2.7 mg/ml) and Amoxicillin (1.8–2.0 mg/ml) by ~9.0–11.5 folds. According to these observations, the SSF improved the SPS-antibacterial activity. This improvement was attributable to an increase in the phenolic compound concentrations and their antioxidant capacity. The protocatechuic, the major phenolic compound found in the FSPS, and the four newly identified phenolic compounds (vanillic, p-coumaric, cinnamic, and quercetin) of FSPS extract have potent antibacterial activities [47, 48]. The highly antioxidant polyphenols not only damage the bacterial cell membrane, influencing its permeability and releasing its intracellular constituents, but they also impair the membrane functions (nutrient uptake, electron transport, protein synthesis, and enzyme activity). As a consequence, these polyphenols have a number of invasive targets, which could lead to bacterial suppression [49, 50]. Many studies demonstrated that the SSF can generate products with upgraded health-related properties from plants and plant residues. The antibacterial properties of phenolics extracted from ginger leaves, curry leaves, garden cress seeds, and chia seeds were also promoted with low MIC results under SSF compared to their unfermented/native forms [15, 17, 18, 34]. In addition, SPS acetone extract showed lower antibacterial activity against E. coli and S. aureus with inhibition zones of ~13 mm and MIC values of 2.1 and 2.0 mg/ml, respectively [6] than that determined in FSPS extract, demonstrating that the SSF significantly enhanced the antibacterial activity of the SP-plant.
Impact of SSF on the SPS-antifungal potency
The antifungal activity was also determined for obtained UFSPS and FSPS phenolic extracts at 50 µg GAE/ml against some human pathogenic fungal resistance strains and is presented in Table 7. The findings demonstrated that the FSPS extract exhibited noticeably higher antifungal activity (P < 0.01) (21–26 mm inhibitory diameters) than the UFSPS extract (16–18 mm) and Econazole (14–15 mm) against all the examined pathogenic fungus strains. In addition, the FSPS exhibited significantly lower (P < 0.01) MIC values against the studied pathogenic fungi, ranging from 0.1–0.2 mg/ml than the UFSPS extract (2.0–2.3 mg/ml) and Econazole (2.6–3.0 mg/ml) by ~11.0–30.0 folds (Table 7). Barakat et al. [6] reported that SPS acetone extract showed antifungal activity against F. proliferatum and P. verrucosum strains with inhibition zones of 16 and 15 mm and MIC values of 2.0 and 2.2 mg/ml, respectively, indicating that the SSF technology considerably increased the antifungal activity in the SP-plant. These findings suggest that the SSF-applied technology greatly enhanced the antifungal activity of the SPS. Similarly, the antifungal properties of garden cress seeds were also elevated with low MIC values under SSF in comparison to their unfermented form [18]. This noticed enhancement is due to an increase in the concentrations of the recognized phenolic compounds and their antioxidant capacity as well as newly discovered phenolic compounds in the FSPS extract. The main phenolic compounds detected in FSPS extract, such as protocatechuic, gallic, p-hydroxybenzoic, caffeic, vanillic, p-coumaric, cinnamic, and quercetin have been demonstrated to have potent antifungal activity [40, 51, 52]. These phenolic compounds have a potent ability to attach to various fungal molecular structures. Many antifungal mechanisms of many phenolic compounds were reported including inhibition/reduction of ergosterol biosynthesis [53, 54], apoptosis induction [55], reduction of the fugal-adherence properties [56], and/or inhibition of some vital fungal enzymes [57]. Therefore, the FSPS-phenolic compounds can be used as a potential antifungal agent and can be also added to food to inhibit the growth of undesired bacteria and fungi, prevent foodborne diseases, and extend shelf-life.
Conclusion
In this work, a statistical approach (central composite design and response surface methodology) was employed to optimize the SSF conditions/variables for the maximum production of phenolic compounds from SPS. The current investigations demonstrated that the TPC, TAA, phenolic compound composition, and antibacterial and antifungal potency of the SPS increased several folds under statistically optimized SSF by T. reesei. As a result, SPS is a promising substrate for obtaining value-added antioxidant phenolic compounds under SSF by T. reesei. The FSPS-phenolic compounds can be added to different foods to develop new bioactive-functional products that target different physiological activities in the body and can also be used as an antimicrobial remedy.
References
A. Scalbert, C. Manach, C. Morand, C. Rémésy, Dietary polyphenols and the prevention of diseases. Crit. Rev. Food. Sci. Nutr. 45, 287–306 (2005)
J.P.E. Spencer, M.M. Abd El Mohsen, A.M. Minihane, J.C. Mathers, Biomarkers of the intake of dietary polyphenols: strengths, limitations, and application in nutrition research. Br. J. Nutr. 99, 12–22 (2008)
A.M. Abdel-Aty, M.B. Hamed, W.H. Salama, M.M. Ali, A.S. Fahmy, S.A. Mohamed, Ficus carica, Ficus sycomorus, and Euphorbia tirucalli latex extracts: phytochemical screening, antioxidant and cytotoxic properties. Biocatal. Agric. Biotechnol. 20, 101199 (2019)
A.Z. Barakat, R.I. Bassuiny, A.M. Abdel-Aty, S.A. Mohamed, Diabetic complications and oxidative stress: the role of phenolic-rich extracts of saw palmetto and date palm seeds. J. Food Biochem. 44, e13416 (2020)
A.M. Abdel-Aty, W.H. Salama, M.B. Hamed, A.S. Fahmy, S.A. Mohamed, Phenolic-antioxidant capacity of mango seed kernels: therapeutic effect against viper venoms. Rev. Bras. Farmacogn. 28, 594–601 (2018)
A.Z. Barakat, A.R. Hamed, R.I. Bassuiny, A.M. Abdel-Aty, S.A. Mohamed, Date palm and saw palmetto seeds functional properties: antioxidant, anti-inflammatory, and antimicrobial activities. J. Food Measur. Character 14, 1064–1072 (2020)
A.M. Abdel-Aty, A.M. Elsayed, H.A. Salah, R.I. Bassuiny, S.A. Mohamed, Egyptian chia seeds (Salvia hispanica L.) during germination: upgrading of phenolic profile, antioxidant, antibacterial properties and relevant enzymes activities. Food Sci. Biotechnol. 30, 723–734 (2021)
A.M. Abdel-Aty, W.H. Salama, A.S. Fahmy, S.A. Mohamed, Impact of germination on antioxidant capacity of garden cress: new calculation for determination of total antioxidant activity. Sci. Hortic. 246, 155–160 (2019)
Grand View Research (2019), https://www.grandviewresearch.com/press-release/global -polyphenols-market
C. Ingrao, N. Faccilongo, L. Di Gioia, A. Messineo, Food waste recovery into energy in a circular economy perspective: a comprehensive review of aspects related to plant operation and environmental assessment. J. Clean. Prod. 184, 869–892 (2018)
T.I. Lafka, A.E. Lazou, V.J. Sinanoglou, E.S. Lazos, Phenolic and antioxidant potential of olive oil mill wastes. Food Chem. 125, 92–98 (2011)
K. Ameer, H.M. Shahbaz, J.H. Kwon, Green extraction methods for polyphenols from plant matrices and their byproducts: a review. Compr. Rev. Food Sci. Food Saf. 16, 295–315 (2017)
I. Ignat, I. Volf, V.I. Popa, A critical review of methods for characterization of polyphenolic compounds in fruits and vegetables. Food Chem. 126, 1821–1835 (2011)
A. Khosravi, S.H. Razavi, The role of bioconversion processes to enhance polyphenol bio-accessibility in rice bio-accessibility of polyphenols in rice. Food Biosci. 35, 100605 (2020)
A.M. Abdel-Aty, A.Z. Barakat, R.I. Bassuiny, S.A. Mohamed, Improved production of antioxidant-phenolic compounds and certain fungal phenolic-associated enzymes under solid-state fermentation of chia seeds with Trichoderma reesei: response surface methodology-based optimization. J. Food Measur. Character. (2022). https://doi.org/10.1007/s11694-022-01447-6
M. Molaverdi, K. Karimi, S. Mirmohamadsadeghi, M. Galbe, High titer ethanol production from rice straw via solid-state simultaneous saccharification and fermentation by Mucor indicus at low enzyme loading. Energy Convers. Manag. 182, 520–529 (2019)
R.M. Saleh, S.A. Kabli, S.M. Al-Garni, M.A. Al-Ghamdi, A.M. Abdel-Aty, S.A. Mohamed, Solid-state fermentation by Trichoderma viride for enhancing phenolic content, antioxidant and antimicrobial activities in ginger. Lett. Appl. Microbiol. 67, 161–167 (2018)
A.M. Abdel-Aty, R.I. Bassuiny, A.Z. Barakat, S.A. Mohamed, Upgrading the phenolic content, antioxidant and antimicrobial activities of garden cress seeds using solid-state fermentation by Trichoderma reesei. J. Appl. Microbiol. 127, 1454–1467 (2019)
N. Abu Yazid, R. Barrena, D. Komilis, A. Sánchez, Solid-state fermentation as a novel paradigm for organic waste valorization: a review. Sustainability 9, 224 (2017)
I. Talhi, L. Dehimat, A. Jaouani, R. Cherfia, M. Berkani, F. Almomani, Y. Vasseghian, N.K. Chaouche, Optimization of thermostable proteases production under agro-wastes solid-state fermentation by a new thermophilic Mycothermus thermophilus isolated from a hydrothermal spring Hammam Debagh. Algeria. Chemosph. 286, 131479 (2022)
M. Suzuki, Y. Ito, T. Fujino, M. Abe, K. Umegaki, S. Onoue, H. Noguchi, S. Yamada, Pharmacological effects of saw palmetto extract in the lower urinary tract. Acta Pharmacol. Sin. 30, 271–281 (2009)
Y.S. Velioglu, G. Mazza, L. Gao, B.D. Oomah, Antioxidant activity and total phenolics in selected fruits, vegetables, and grain products. J. Agric. Food Chem. 46, 4113–4117 (1998)
K.H. Kim, R. Tsao, R. Yang, S.W. Cui, Phenolic acid profiles and antioxidant activities of wheat bran extracts and the effect of hydrolysis conditions. Food Chem. 95, 466–473 (2006)
C. Ao, A. Li, A.A. Elzaawely, T.D. Xuan, S. Tawata, Evaluation of antioxidant and antibacterial activities of Ficus microcarpa L. fil. extract. Food Control 19, 940–948 (2008)
R. Re, N. Pellegrini, A. Proteggente, A. Pannala, M. Yang, C. Rice-Evans, Antioxidant activity applying an improved ABTS radicalcation decolorization assay. Free Radic. Biol. Med. 26, 1231–1237 (1999)
A.W. Bauer, W.M. Kirby, J.C. Sherris, M. Turk, J. Am, Antibiotic susceptibility testing by a standardized single disk method. Clin. Pathol. 44, 493 (1996)
National Committee for Clinical Laboratory Standards, Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, approved standard M7–A7, vol. 26 (Clinical and Laboratory Standards Institute, Wayne, PA, 2009)
N. Liu, M. Song, N. Wang, Y. Wang, R. Wang, X. An, J. Qi, The effects of solid-state fermentation on the content, composition and in vitro antioxidant activity of flavonoids from dandelion. PLoS ONE 15, e0239076 (2020)
H.M. Abdel-Mageed, A.Z. Barakat, R.I. Bassuiny, A.M. Elsayed, H.A. Salah, A.M. Abdel-Aty, S.A. Mohamed, Biotechnology approach using watermelon rind for optimization of α-amylase enzyme production from Trichoderma virens using response surface methodology under solid-state fermentation. Folia Microbiol. 67, 253–326 (2022)
S.A. Mohamed, A.L. Al-Malki, J.A. Khan, S.A. Kabli, S.M. Al-Garni, Solid state production of polygalacturonase and xylanase by Trichoderma species using cantaloupe and watermelon rinds. J. Microbiol. 51, 605–611 (2013)
C.G. Schmidt, L.M. Gonçalves, L. Prietto, H.S. Hackbart, E.B. Furlong, Antioxidant activity and enzyme inhibition of phenolic acids from fermented rice bran with fungus Rizhopus oryzae. Food Chem. 146, 371–377 (2014)
L.N. Hlahla, F.N. Mudau, I.K. Mariga, Effect of fermentation temperature and time on the chemical composition of bush tea (Athrixia phylicoides DC). J. Med. Plants Res. 4, 824–829 (2010)
R.K. Salar, M. Certik, V. Brezova, Modulation of phenolic content and antioxidant activity of maize bysolid state fermentation with Thamnidium elegans CCF1456. Biotechnol. Bioprocess. Eng. 17, 109–116 (2012)
H.A. Salah, R.I. Bassuiny, M.I. El-Khonezy, A.S. Fahmy, S.A. Mohamed, Impact of solid-state fermentation by Trichoderma spp. on phenolic content, antioxidant and antibacterial activities of curry leaf powder. J. Food Measur. Character 13, 1333–1340 (2019)
N. Ritthibut, S. Oh, S. Lim, Enhancement of bioactivity of rice bran by solid-state fermentation with Aspergillus strains. LWT-Food Sci. Technol. 135, 110273 (2021)
A.Y. Ibrahim, S.A. El-Newary, M.A. El-Raey, Evaluation of the antioxidant, anti-inflammatory, and antitumor properties of Sabal grown in Egypt. Egypt Pharmac. J. 16, 168–183 (2018)
L. Liu, W. Wen, R. Zhang, Z. Wei, Y. Deng, J. Xiao et al., Complex enzyme hydrolysis releases antioxidative phenolics from rice bran. Food Chem. 214, 1–8 (2017)
A. Starzynska-Janiszewska, B. Stodolak, R. Socha, B. Mickowska, A. Wywrocka-Gurgul, Spelt wheat tempe as a value-added whole-grain food product. LWT-Food Sci. Technol. 113, 108250 (2019)
Y. Xiao, G. Xing, X. Rui, W. Li, X. Chen, M. Jiang, Enhancement of the antioxidant capacity of chickpeas by solid state fermentation with Cordyceps militaris SN-18. J. Funct. Foods 10, 210–222 (2014)
S. Kakkar, S. Bais, A Review on protocatechuic acid and its pharmacological potential. ISRN Pharmacol. 12, 952943 (2014)
S. Cheemanapalli, R. Mopuri, R. Golla, C.M. Anuradha, S.K. Chitta, Syringic acid (SA)‒a review of its occurrence, biosynthesis, pharmacological and industrial importance. Biomed. Pharmacother. 108, 547–557 (2018)
S.J. Hur, S.Y. Lee, Y.-C. Kim, I. Choi, G.-B. Kim, Effect of fermentation on the antioxidant activity in plant-based foods. Food Chem. 160, 346–356 (2014)
Z. Zhang, G. Lv, H. Pan, L. Fan, C.R. Soccol, A. Pandey, Production of powerful antioxidant supplements via solid-state fermentation of wheat (Triticum aestivum Linn.) by Cordyceps militaris. Food Technol. Biotechnol. 50, 32–39 (2012)
J. Moore, Z. Cheng, J. Hao, G. Guo, J.G. Liu, C. Lin, L.L. Yu, Effects of solid-state yeast treatment on the antioxidant properties and protein and fiber compositions of common hard wheat bran. J. Agric. Food Chem. 55, 10173–10182 (2007)
J.H. Yang, Y.H. Tseng, Y.L. Lee, J.L. Mau, Antioxidant properties of methanolic extracts from monascal rice. LWT-Food Sci. Technol. 39, 740–747 (2006)
T. Bhanja Dey, S. Chakraborty, K.K. Jain, A. Sharma, R.C. Kuhad, Antioxidant phenolics and their microbial production by submerged and solid-state fermentation process: a review. Trends Food Sci. Technol. 53, 60–74 (2016)
M.J. Alves, I.C.F.R. Ferreira, H.J.C. Froufe, R.M.V. Abreu, A. Martins, M. Pintado, Antimicrobial activity of phenolic compounds identified in wild mushrooms, SAR analysis and docking studies. J. Appl. Microbiol. 115, 346–357 (2013)
Y. Semaming, P. Pannengpetch, S.C. Chattipakorn, N. Chattipakorn, Pharmacological properties of protocatechuic acid and its potential roles as complementary medicine. Evidence-Based Complement Altern. Med. (2015). https://doi.org/10.1155/2015/593902
Y.S. Chan, K.-P. Chong, Bioactive compounds of Ganoderma boninense inhibited methicillin-resistant Staphylococcus aureus growth by affecting their cell membrane permeability and integrity. Molecules 27, 838 (2022)
F. Nourbakhsh, M. Lotfalizadeh, M. Badpeyma, A. Shakeri, V. Soheili, From plants to antimicrobials: natural products against bacterial membranes. Phytother. Res. 36, 33–52 (2022)
H.O. Elansary, A. Szopa, P. Kubica, H. Ekiert, M.A. Mattar, M.A. Al-Yafrasi, D.O. El-Ansary, T.K. Zin El-Abedin, K. Yessoufou, Polyphenol profile and pharmaceutical potential of Quercus spp. bark extracts. Plants 8, 486 (2019)
H.O. Elansary, A. Szopa, M. Klimek-Szczykutowicz, H. Ekiert, A.A. Barakat, F.A. Al-Mana, Antiproliferative, antimicrobial, and antifungal activities of polyphenol extracts from Ferocactus species. Processes 8, 138 (2020)
T.A. Bitencourt, T.T. Komoto, B.G. Massaroto, C.E.S. Miranda, R.O. Beleboni, M. Marins, A.L. Fachin, Trans-chalcone and quercetin down-regulate fatty acid synthase gene expression and reduce ergosterol content in the human pathogenic dermatophyte Trichophyton rubrum. BMC Complement. Altern. Med. 13, 229 (2013)
Z.J. Li, M. Liu, G. Dawuti, Q. Dou, Y. Ma, H.G. Liu, S. Aibai, Antifungal activity of gallic acid in vitro and in vivo. Phytother. Res. 31, 1039–1045 (2017)
J. Lee, D.G. Lee, Novel antifungal mechanism of resveratrol: apoptosis inducer in Candida albicans. Curr. Microbiol. 70, 383–389 (2015)
M. Feldman, S. Tanabe, A. Howell, D. Grenier, Cranberry proanthocyanidins inhibit the adherence properties of Candida albicans and cytokine secretion by oral epithelial cells. BMC Complement. Altern. Med. 12, 6 (2012)
H.L. Cheah, V. Lim, D. Sandai, Inhibitors of the glyoxylate cycle enzyme ICL1 in Candida albicans for potential use as antifungal agents. PLoS ONE 9, e95951 (2014)
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Abdel-Aty, A.M., Barakat, A.Z., Bassuiny, R.I. et al. Antioxidant-polyphenols of saw palmetto seeds: statistical optimized production and improved functional properties under solid-state fermentation by Trichoderma reesei. Food Measure 17, 1132–1143 (2023). https://doi.org/10.1007/s11694-022-01675-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11694-022-01675-w