Abstract
Chia seeds (CS) are becoming increasingly consumed due to their great nutritional and therapeutic properties. In this study, solid-state fermentation (SSF) of CS by Trichoderma reesei was employed to maximize the production of the antioxidant-phenolic compounds and some fungal phenolic-associated enzymes (α-amylase, xylanase, β-glucosidase, polygalacturonase, and phenylalanine ammonia-lyase). The SSF-conditions were statistically optimized using response surface methodology (RSM). In the statistical model, four variables were analyzed at two levels. According to RSM, the adjusted R2 (< 0.9) is reasonably consistent with the predicted R2 (< 0.9), indicating that the statistical model is valid. The optimal conditions for maximum production of both phenolic compounds and fungal phenolic-associated enzymes were found to be 28 °C, pH 7.0, 20% moisture, and 7-day fermentation. The total phenolic content of fermented CS (FCS) increased 23 folds and total antioxidant activity was enhanced by 113- and 150-fold using DPPH and ABTS methods, respectively. Three new phenolics (kaempferol, apigenin, and p-coumaric) were recognized in FCS using HPLC analysis. The activities of all the extracted phenolic-associated enzymes showed strong correlations with the phenolic content of FCS. Against some human-pathogenic bacteria, FCS extract displayed considerably better antibacterial activity than UFCS extract. Finally, the phenolic-rich-FCS can be employed as a dietary supplement as well as an antibacterial agent. Furthermore, T. reesei has produced considerable quantities of industrially valuable enzymes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The demand for natural antioxidant-phenolic compounds is mainly increasing due to their effectiveness in the treatment of various diseases including cancer, cardiovascular and neurodegenerative disorders, diabetes, and high cholesterol [1,2,3]. These active compounds have been linked to a variety of health benefits, making a regular diet rich in antioxidant-phenolic compounds essential for health promotion. [4]. Natural antioxidant-phenolic compounds are also used in a variety of industries, including food, beverages, and pharmaceuticals [3]. Many methods have been used to extract phenolic compounds from plants and plant-based materials. Because phenolic compounds are usually found in insoluble forms that are conjugated with sugars and linked to the cell wall by many bonds, a poor yield of these compounds was obtained during the extraction process. [5]. Therefore, they cannot be completely extracted by conventional methods that used organic solvents. Additionally, the possibility of solvent contamination during food processing, as well as the high cost of organic solvents, encouraged the development of alternative clean techniques [6].
Solid-state fermentation (SSF) by fungi, as a clean biological treatment, can be used for extraction of conjugated/bound phenolic compounds from plants and plant based-materials. During the fermentation process, SSF-fungi could produce mass amounts of hydrolytic enzymes, which can easily break down conjugated phenolic compounds and convert insoluble phenolic compounds into free-soluble forms. In addition, the fungal synthesized enzymes (amylase, xylanase, cellulases, β-glucosidase, protease, and pectinase) have great commercial values and are extensively used in food industries [7]. Furthermore, the SSF enhanced various bioactive activities of plants and plant based-materials [8,9,10]. Phenylalanine ammonia-lyase has recently received a lot of attention and is considered to be an important therapeutic enzyme with a wide range of applications in medicine. It is mainly found in plants, algae, and certain microorganisms and is absent in humans. Microbial PAL is crucial for the production of industrially important secondary metabolites including antioxidant-phenolic compounds. Microbial PAL was obtained from a few microorganisms including Rhodotorula glutinis and Anabaena variabilis [11]. However, there was no information about the production of PAL from Trichoderma reesei.
Chia (Salvia hispanica L., family: Lamiaceae) originated from Mexico and currently is cultivated all over the world as a dietary supplement and for medicinal purposes. Chia seeds have a great nutritional value as they are a rich source of proteins, carbohydrates, ash, lipids, and dietary fibers [12]. In addition, chia seeds contained many valuable antioxidant-phenolic compounds and were incorporated in different foods and beverages as functional food [13, 14]. The European Parliament approved the incorporation of chia seeds in different food products in various countries [12]. Furthermore, chia seeds were incorporated in gluten-free products for helping people suffering from celiac disease and wheat allergy [15]. Chia seeds also inhibited cholinesterase activity, prevented neurodegenerative diseases, and improved insulin sensitivity and lipid metabolism [16, 17].
The germination process recently upgraded/enhanced the concentrations of phenolic compounds and the biological activities of chia seeds. [18]. However, no studies were reported about the impact of SSF on the phenolic compounds and their functional properties of the Egyptian chia seeds. In this study, response surface methodology (RSM) was utilized to optimize SSF-conditions in order to maximize the production of phenolic compounds from Egyptian chia seeds by Trichoderma reesei and analyze their functional properties. The maximum production of fungal phenolic-associated enzymes (α-amylase, xylanase, β-glucosidase, polygalacturonase, and PAL), which are regarded as industrially important enzymes, was also optimized.
Materials and methods
Materials
The Egyptian chia seeds were got from Agriculture Research Center, Giza, Egypt. They were washed and oven-dried (at 40 °C) before being ground. Trichoderma reesei was obtained from the department of microbial chemistry, National Research Centre (NRC, Giza, Egypt). The obtained strain was grown on potato dextrose agar (PDA) for 7 days at 28 °C. Human pathogenic bacteria (Pseudomonas aeruginosa NRRL B-272, Salmonella typhi ATCC 15,566, Escherichia coli O157-H7 ATCC 51,659, Staphylococcus aureus ATCC 13,565, and Bacillus subtilis BTN7A) were got from the department of toxins and contaminants (NRC, Giza, Egypt). 1,1-diphenyl-2-picrylhydrazyl (DPPH), 2,2-azino-bis (3-ethylbenzo-thiazoline-6-sulfonic acid) (ABTS), Folin Ciocalteu reagent, substrates of enzymes, standards, and chemicals were bought from Sigma-Aldrich Co.
Fermentation conditions and experimental design
Chia seed powder (CS) was air-dried after being sterilized with 70% ethanol. In a 100-ml Erlenmeyer flask, 2 g of sterile CS was mixed with fresh Trichoderma reesei spores (5.0 × 108 CFU/ml). Response surface methodology (RSM) and central composite design (CCD) were used to optimize SSF-conditions/parameters (temperature, pH, moisture, and fermentation time) (Factorial design expert V11 software). The levels of SSF-parameters were chosen at temperature (28 and 32 °C), pH (6 and 7), moisture (15 and 20%), and fermentation time (6 and 7 days). Sixteen experiments were carried out, with the results based on the average of three independent experiments.
Extraction of phenolic compounds and enzymes
Fermented chia seeds (FCS) were dispersed in either 80% methanol or distilled water at a ratio of 1:20 (W/V) in both cases. They were also incubated overnight at 28 °C with 100 rpm shaking. The extracts were then filtered twice using filter paper Whatman-1. The obtained extracts were phenolic FCS and enzymatic FCS for methanol and distilled water, respectively.
Total phenolic measurement
The method of Velioglu et al. [19] was used to measure the content of phenolics. A reaction mixture containing 100 µl of methanol extract, 800 µl of distilled water, and 100 µl of Folin Ciocalteu reagent was incubated for 5 min. After that, 500 µl of 20% Na2CO3 was added and incubated for 30 min. At A750 nm, the absorbance was measured. Phenolic content is expressed as mg gallic acid equivalent (GAE).
Phenolic compound composition
HPLC was used to analyze the phenolic compounds using Agilent Technologies1100 series liquid chromatography [20]. The mobile-phase gradient was a 10: 90 ratio of solvent (2% acetic acid) to solvent (acetonitrile) with a flow rate of 1 ml/min. Peaks at 280, 320, and 360 nm were recorded and identified using corresponding retention times.
Antioxidant activity assays
DPPH assay: in a one ml reaction mixture, 25 µl methanol extract was combined with DPPH reagent dissolved in methanol (0.1 mM) and incubated for 30 min in the dark. [21]. At A517 nm, the absorbance was measured. The DPPH radical-scavenging activity % = (Control absorbance − sample absorbance/control absorbance) ×100.
ABTS assay: 10 µl methanol extract was combined with ABTS reagent in a one ml reaction mixture and incubated for 1 min [22]. At A734 nm, the absorbance was measured. The ABTS radical-scavenging activity % was calculated as DPPH assay.
Enzymatic assays
Miller method [23] was used to investigate the activities of α-amylase, xylanase, and polygalacturonase. For α-amylase, xylanase, and polygalacturonate, the substrates were starch, xylan, and polygalacturonic acid, respectively. The enzymatic extract (100 µl) and 5 mg substrate were combined together in 0.05 M sodium acetate buffer, pH 5.5, and incubated at 37 °C for one h. Then 500 µl of dinitro-salicylic acid was added and the mixture was boiled for ten min. The mixture was measured at A560 nm after cooling. The enzyme concentration that liberates one µmol of reducing sugar per hour is defined as one unit of enzyme activity.
According to Gunata et al. [24], the activity of β-Glucosidase was investigated. One ml of a solution containing 100 µl of p-nitrophenol ß-D-glucopyranoside (9 mM), 50 mM sodium acetate buffer, pH 6.0, and 100 µl of the enzymatic extract was incubated for one h at 30 °C. Then half ml 20% sodium carbonate was added. The absorbance was recorded at A400 nm. The enzyme concentration that liberates one µmol of p-nitrophenol per hour is defined as one unit of enzyme activity.
PAL activity was examined according to Sirin et al. [25]. A mixture of one ml containing 40 mM phenylalanine, 100 mM Tris-HCl buffer, pH 8.5, and 100 µl enzymatic extract was incubated for one h. One unit of enzyme activity is described as a 1.0 OD increase at A290 nm.
Antibacterial activity
To evaluate the antibacterial activity of the FCS compared to unfermented chia seeds (UFCS), a well-diffusion method was used [26]. On Mueller–Hinton agar plates, each bacterium suspension (100 µl) was dispersed at a concentration of 108 CFU/ml. Wells were filled with FCS and UFCS extracts (50 µg GAE). Then incubation was carried out at 37 °C for 18 h. The diameters of inhibitory zones were measured to determine antibacterial activity. According to National Committee for Clinical Laboratory Standards [27], the minimum inhibition concentration (MIC) was determined using the agar dilution-diffusion method. On Mueller–Hinton agar, each bacterial strain was distrusted at a concentration of 108 CFU/ml. FCS and UFCS extracts in various doses (0.1-4.0 mg/ml) were applied to inoculated agar wells and incubated for 18 h at 37 °C. The lowest concentration at which bacteria cannot grow is MIC.
Statistical analysis
The SSF conditions were optimized and analyzed by RSM and CCD of factorial design expert V11 software (Stat-Ease Inc., US). Sixteen experiments were carried out and the statistical results, models, and equations were validated using ANOVA based on the p-value with a 95% level of confidence. The rest of the data was analyzed using one-way ANOVA and a Tukey post-test (GraphPad Prism 5 software). All measurements were expressed as means ± SD (n = 4).
Results and discussion
Optimization of SSF-parameters of chia seeds using RSM
Herein, we study the influence of the SSF process by T. reesei on the phenolic content, antioxidant activity, and functional properties of chia seeds (CS) as well as enzymes responsible for liberating and producing phenolic compounds. Optimizing of SSF-parameters is a very complex process due to these parameters interacting together simultaneously. So, many multivariance-analytical methods were used to adjust the SSF-parameters. In this study, the RSM and CCD were employed to find the best SSF-parameters for maximum phenolic compound and fungal phenolic-associated enzymes production. Fermentation time (A), pH (B), temperature (C), and moisture (D) were among the SSF-parameters, and their proper ranges/levels were determined using single-parameter preliminary experiments. One of these parameters was changed while the other parameters remain constant in each experiment (data not shown).
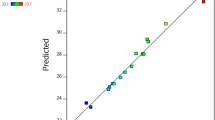
Table 1 summarizes the SSF-parameters of CS as well as actual and predicted values of phenolic contents and the activities of some fungal phenolic-associated enzymes using the experimental design for sixteen runs. The validation of obtained responses was checked using the statistical models and ANOVA analysis and presented in Table 2. Two-factor interaction (2FI) model was suggested for all responses (phenolic content and enzymatic activities). According to the findings, the adjusted R2 is reasonably consistent with the predicted R2. Additionally, the F-value and P-values obtained from the suggested model are significant for all responses. Furthermore, the signal-to-noise ratio ‘Adeq accuracy’ for all responses is greater than 4, which is preferred (Table 2). Moreover, the actual and predicted values for all responses were very close to the expected line (Fig. 1), demonstrating the accuracy of the established model.
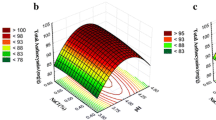
Table 3; Fig. 2 illustrate the impacts of the investigated parameters (fermentation time, pH, moisture, and temperature) and their interactions on the responses’ values. The results showed that the fermentation time (A), pH (B), and moisture (D) have a positive impact/favorable effect on all responses. While the fermentation temperature (C) at a high level, has a negative impact on all responses compared to its low level. The mutual interaction between parameters was shown differently in each response. For phenolic content, there was a significant positive (p < 0.05) interaction between AB, AC, and AD. There was also a significant positive (p < 0.05) interaction between A and D in all responses. Further, the negative effect of temperature (C) on the β-glucosidase production was reversed by interaction with time (A), whereas, the interaction between pH (B) and moisture (D) was changed from positive to negative (Table 3). By the analysis, the equations of the model were described as follow:
Phenolic content = + 25.26 + 0.7986 A + 0.8611B-0.8764 C + 0.7396 D + 0.1986 AB -0.1639 AC + 0.1521 AD -0.1514 BC + 0.0896 BD -0.0979 CD.
α-amylase = + 403.80 + 20.17 A + 23.93 B-1.90 C + 24.68 D + 0.0560AB-0.0274 AC + 0.0549 AD-0.0481 BC + 0.0506 BD-0.0410 CD.
Xylanase = + 428.15 + 10.89 A + 15.89 B-6.32 C + 17.58 D + 0.0057 AB − 0.0557 AC + 0.0925 AD -0.1580 BC + 0.1698 BD -0.2438 CD.
Poly-galacturonase = + 611.13 + 21.13 A + 19.26 B-6.93 C + 13.95 D + 0.0131 AB-0.0567 AC + 0.07350 AD-0. 0547 BC + 0.0715 BD-0.0338 CD.
β-glucosidase = + 507.64 + 6.94 A + 29.67 B-9.44 C + 9.63 D + 0.0500 AB + 0.0623 AC + 0.0595 AD-0.0499 BC-0.0783 BD-0.0656 CD.
Phenylalanine ammonia lyase = + 1690.38 + 96.00 A + 54.61 B -18.76 C + 120.25 D-0.0001 AB-0.0006 AC + 0.0141 AD + 0.0130 BC + 0.0006 BD + 0.0011 CD.
The predicted maximum values of phenolic content and the activities of tested enzymes as well as the optimal SSF-parameters were estimated by using numerical maximization and approaches of desirability function (Fig. 3). The predicted maximum responses were very close to the actual responses with a desirability function of 0.998 as shown in Table 4. In addition, low relative error percentages (2.03-0.0%) between predicted and actual values of all responses were observed referring to the fitting and accuracy of the model and experimental design used. Similarly, the RSM was recently used to optimize the SSF-parameters for the production of phenolics and flavonoids from garden cress seeds and dandelion, respectively [10, 28]. The RSM was also used to optimize the SSF-parameters for the production of α-amylase, xylanase, and polygalacturonase from garden cress seeds and watermelon rind by T. reesei and T. virens, respectively [10, 29].
Overall, 28 °C, pH 7.0, 20% moisture, and a 7-day fermentation time were found to be the best SSF conditions for the production of both phenolic compounds and all of the examined fungal phenolic-associated enzymes from FCS. In general, the choice of physiological parameters has a significant impact on the efficiency of the SSF process. High moisture content can reduce the porosity of particles and their intermolecular structures. While the nutrition and growth of the microorganisms were limited by low moisture content. Additionally, changes in pH diminished the release of microbial enzymes and the ability of antioxidant-phenolic compounds to donate electrons. [30]. Furthermore, a high fermentation temperature decreased microbial growth, increased molecule mobility, and accelerated phenolic compound decomposition. Low moisture content (20–30%), low temperature (28–30 °C), fermentation time (5–7 days), and pH (6.0–7.0) were previously detected as the best SSF-parameters for the production of phenolic compounds from ginger, garden cress seeds, bush tea, and maize [9, 10, 31, 32]. When SSF was performed at temperatures higher than 30 °C, the lowest levels of phenolics were obtained from ginger [9] and bush tea [31].
The total phenolic content increased by 23-fold (p < 0.01) from 1.3 mg GAE g− 1 in the UFCS extract to 30.0 mg GAE g− 1 in the FCS extract (Table 5). Similarly, the total phenolic content of some plants and plant-based materials such as wheat bran, wheat grain, oat, buckwheat, barley, rye, rice, and rice bran, was enhanced several folds under SSF by fungi [33,34,35,36,37,38]. The phenolic content of ginger and garden cress seeds, both medicinal plants, was also enhanced by 6 and 9 folds, respectively, under SSF by T. viride and T. reesei [9, 10]. Furthermore, the observed phenolic content of FCS (30.0 mg GAE g-1) was three times higher than the phenolic content of 7-day CS sprouts (9.0 mg GAE g-1) [18], demonstrating that the SSF process is more efficient than the germination process for enhancing CS phenolic content.
Production of fungal enzymes
Most plant phenolic compounds are bound/conjugated with one or more derivatives of sugar molecules. These sugar molecules bind to the hydroxyl groups of phenolic compounds and cause a reduction in their antioxidant activity. The presence of free hydroxyl groups on the phenolic ring increases antioxidant activity and helps to scavenge free radicals. Fungi use both simple and complex molecules for growing through the secretion of a variety of extracellular enzymes. Some fungal-synthesized enzymes break down the lignocellulosic cell walls of the plant, converting conjugated phenolic compounds into free phenolic compounds, leading to the liberation of various free-phenolic compounds [8, 10, 39]. However, various antioxidant-phenolic compounds are bio-synthesized by the action of the PAL enzyme [40, 41]. Therefore, in this study, the activities of some fungal phenolic-associated enzymes: β-glucosidase (563 U g-1 seed), xylanase (480 U g-1 seed), α-amylase (475 U g-1 seed), and polygalacturonase (473 U g-1 seed) and PAL (1980 U g-1 seed) were investigated and their relations with phenolic compounds variation were also studied during the SSF. All of the examined fungal phenolic-associated enzymes and phenolic compounds were produced to their maximum potential at the same optimal SSF-conditions (28 °C, pH 7.0, 20% moisture, and 7 days of fermentation). Additionally, strong correlations between phenolic content and the activity of examined enzymes were observed, with R2 values ranging from 0.84-to 0.92. (Fig. 4). This finding suggests that the examined enzymes are essential in increasing phenolic content by liberating/producing free phenolic compounds and improving antioxidant activity. Under SSF, the activities of α-amylase, xylanase, polygalacturonase, and β-glucosidase were increased and linked to the production of phenolic compounds from ginger, garden cress seeds, soy germ, and wheat [9, 10, 39, 42]. There have been few studies on PAL enzyme production under SSF. Bacillus subtilis produced the PAL enzyme (58 U/ml) from agro-industrial wastes under SSF at 30 °C and 96 h of fermentation [43]. These findings indicate that the examined fungal phenolic-associated enzymes produced under SSF of CS could be employed in the food and pharmaceutical industries.
HPLC analysis
The HPLC technique was used to identify the phenolic compound composition of the UFCS and FCS phenolic extracts, as shown in Table 6. The UFCS phenolic extract contained twelve phenolic compounds with concentrations ranging from 0.008 to 0.250 mg/g DW. In the FCS phenolic extract, fifteen phenolic compounds were identified with concentrations ranging from 0.16 to 2.1 mg/g DW. Three new phenolics (p-coumaric, apigenin, and kaempferol) were recognized in FCS extract. The contents of all investigated phenolic compounds in FCS extract were 6.0 to 210 times greater (p < 0.01) than in UFCS extract. The main phenolics recognized in both CS extracts were protocatechuic, caffeic, and rosmarinic with greater quantities in FCS extract (1.85, 1.80, and 1.50 mg/g) than in UFCS extract (0.20, 0.22, and 0.25 mg/g DW). Additionally, p-hydroxybenzoic acid levels in FCS extract (2.10 mg/g DW) were 210 times higher than in UFCS extract (0.01 mg/g DW). This enhancement in phenolic compounds concentrations and the production of new phenolic compounds could be attributed to the action of fungal phenolic-associated enzymes released during SSF. These enzymes break down plant cell walls, converting many insoluble/conjugated phenolic compounds to soluble/free phenolic compounds or producing new phenolic compounds [44]. Ferulic, sinapic, and coumaric acids increased 63, 24, and 20 times in fermented rice bran under SSF, respectively, compared to unfermented rice bran. Also, caffeic acid was found only in fermented rice bran and not in unfermented rice bran [38]. Pyrogallol, gallic acid, and coumaric acid were also raised 65, 62, and 36 times, respectively, while apigenin was only found in fermented garden cress seeds extract over unfermented extract [10]. Interestingly, the majority of the phenolic compounds found in FCS extract contain a variety of bioactive properties, including antioxidant, antibacterial, anti-snake, anticancer, anti-inflammatory, and antidiabetic [1, 2, 10, 45,46,47,48]. As a consequence, these phenolic compounds can be utilized as dietary supplements to reap their health benefits and to treat a variety of critical diseases.
Antioxidant capacity of SC phenolic extracts
The antioxidant activity of UFCS and FCS was investigated using the DPPH and ABTS assays under SSF-optimal conditions. The DPPH and ABTS IC50 values for UFCS extract (21.7 and 13.3 µg GAE/ml, respectively) fell considerably (p < 0.01) to (4.4 and 2.0 µg GAE/ml, respectively) for FCS extract (Table 5). Additionally, when total antioxidant activity was measured using IC50 of DPPH and ABTS, the results increased considerably (p < 0.01) from (60 and 100, respectively) for UFCS extract to (6,818 and 15,000, respectively) for FCS extract by ~ 113 and 150 folds, respectively as shown in Table 5. The antioxidant activity of FCS extract was found to be highly associated with its phenolic content. As a result, the increased antioxidant activity in FCS is attributed to a significant increase in phenolic content. Besides, the majority of the phenolic compounds found in FCS are recognized to be powerful antioxidants. Furthermore, greater β-glucosidase activity is associated with higher FCS phenolic content, resulting in phenolic compound breakdown and aglycones liberation. Aglycones have high antioxidant activity and are used in a variety of foods and beverages [30]. Increased PAL enzyme production may also help in the synthesis of phenolic compounds with antioxidant properties [49]. Likewise, SSF improved the antioxidant capacity of many plants and plant-based materials such as rice [50], oat [51], black and soya bean [37], ginger [9], and garden cress [10]. In addition, Abdel-Aty et al. [18] observed that the total antioxidant activity of 7-day chia sprouts increased 10- and 17-fold using DPPH and ABTS free radicals, respectively which was lower than that obtained from FCS.
Antibacterial activity of the CS phenolic extract
The antibacterial efficiency of UFCS and FCS phenolics (50 µg GAE/ml) against five human-enteric pathogenic bacteria was investigated. Amoxicillin at 50 µg was used as a positive control. The FCS phenolic extract displayed considerably better (p < 0.01) antibacterial activity against the studied bacteria, with inhibition diameters ranging from 20.0 to 30.0 mm in comparison to the UFCS (3.0–5.0 mm) and Amoxicillin (5.0–18.0 mm) (Table 7). Additionally, the FCS phenolic extract demonstrated considerably lower (p < 0.01) MIC values against the studied bacteria, ranging from 0.2 to 0.32 mg/ml, as compared to UFCS (2.3-3.0 mg/ml) and Amoxicillin (0.9–2.7 mg/ml) (Table 7). SSF improved the antibacterial activity of CS, according to these findings. This improvement was related to increased levels of phenolic compounds and their antioxidant capacity, such as p-hydroxybenzoic, protocatechuic, caffeic, and rosmarinic, as well as newly found phenolics (apigenin, kaempferol, and p-coumaric) in FCS extract. Some of these phenolics caused bacterial oxidative damage by producing hydrogen peroxide, which damaged the bacterial cell wall and/or impeded bacterial growth. In addition, several investigations have found that some phenolics enhance the action of antibiotics [52,53,54,55]. Furthermore, PAL-produced phenolic compounds have potent antimicrobial activity [11]. Likewise, the antibacterial activity of fermented turmeric [56], ginger [9], garden cress seeds [10], and curry leaf [57] was enhanced with lower MIC values against some pathogenic bacteria compared to their unfermented extracts.
Conclusions
Here, the SSF-conditions of CS were efficiently optimized using RSM to produce the best possible amounts of phenolic compounds and some fungal phenolic-associated enzymes (α-amylase, xylanase, β-glucosidase, polygalacturonase, and PAL). The results clearly indicated that the SSF of CS by T. reesei greatly enhanced the phenolic content, phenolic compound composition, antioxidant and antibacterial activities as well as the activities of some phenolic-associated enzymes, which are considered industrially important enzymes. Hence, FCS extract is considered a rich source of antioxidant-phenolic compounds and can be used to make functional foods and as an antibacterial agent. Furthermore, commercially valuable enzymes were abundantly produced and could be used in the food industry.
References
A.M. Abdel-Aty, M.B. Hamed, W.H. Salama, M.M. Ali, A.S. Fahmy, S.A. Mohamed. (2019) Ficus carica, Ficus sycomorus and Euphorbia tirucalli latex extracts: Phytochemical screening, antioxidant and cytotoxic properties. Biocatalysis and Agriculture Biotechnology 20: 101199
A.Z. Barakat, R.I. Bassuiny, A.M. Abdel-Aty, S.A. Mohamed, Diabetic complications and oxidative stress: The role of phenolic-rich extracts of saw palmetto and date palm seeds. J. Food Biochem. 44, e13416 (2020)
B.R. Albuquerque, S.A. Heleno, M.B.P.P. Oliveira, L. Barros, I.C.F.R. Ferreira, Phenolic compounds: Current industrial applications, limitations and future challenges. Food and Function 12, 14–29 (2021)
S.A. Mohamed, M.A. Awad, E.F. El-Dengawy, H.M. Abdel-Mageed, M.O. El-Badry, H.A. Salah, A.M. Abdel-Aty, A.S. Fahmy, Total phenolic and flavonoid contents and antioxidant activities of sixteen commercial date cultivars grown in Saudi Arabia. RSC Adv. 6, 44814–44819 (2016)
T.B. Dey, R.C. Kuhad, Enhanced production and extraction of phenolic compounds from wheat by solid-state fermentation with Rhizopus oryzae RCK2012. Biotechnol. Rep. 4, 120–127 (2014)
K. Ameer, H.M. Shahbaz, J.H. Kwon, Green extraction methods for polyphenols from plant matrices and their byproducts: a review. Compr. Rev. Food Sci. Food Saf. 16, 295–315 (2017)
S.A. Mohamed, A.L. Al-Malki, J.A. Khan, S.A. Kabli, S.M. Al-Garni, Solid state production of polygalacturonase and xylanase by Trichoderma species using cantaloupe and watermelon rinds. J. Microbiol. 51, 605–611 (2013)
F.V. Dulf, D.C. Vodnar, E.H. Dulf, A. Pintea, Phenolic compounds, flavonoids, lipids and antioxidant potential of apricot (Prunus armeniaca L.) pomace fermented by two filamentous fungal strains in solid state system. Chem. Cent. J. 11, 92 (2017)
R.M. Saleh, S.A. Kabli, S.M. Al-Garni, M.A. Al-Ghamdi, A.M. Abdel-Aty, S.A. Mohamed, Solid-state fermentation by Trichoderma viride for enhancing phenolic content, antioxidant and antimicrobial activities in ginger. Lett. Appl. Microbiol. 67, 161–167 (2018)
A.M. Abdel-Aty, R.I. Bassuiny, A.Z. Barakat, S.A. Mohamed, Upgrading the phenolic content, antioxidant and antimicrobial activities of garden cress seeds using solid-state fermentation by Trichoderma reesei. J. Appl. Microbiol. 127, 1454–1467 (2019)
A. Kawatra, R. Dhankhar, A. Mohanty, P. Gulati, Biomedical applications of microbial phenylalanine ammonia lyase: Current status and future prospects. Biochimie 177, 142–152 (2020)
R. Ullah, M. Nadeem, A. Khalique, M. Imran, S. Mehmood, A. Javid, J. Hussain, Nutritional and therapeutic perspectives of Chia. (Salvia hispanica L.): A review. J. Food Sci. Technol. 53, 1750–1758 (2016)
V. Zettel, B. Hitzmann, Applications of chia (Salvia hispanica L.) in food products. Trends in Food Science and Technology 80, 43–50 (2018)
J. Cotabarren, A.M. Rosso, M. Tellechea, J. Garcia-Pardo, J.L. Rivera, W.D. Obregon, M.G. Parisi, Adding value to the chia (Salvia hispanica L.) expeller: Production of bioactive peptides with antioxidant properties by enzymatic hydrolysis with papain. Food Chem. 274, 848–856 (2019)
S.D. Maidana, S. Finch, M. Garro, G. Savoy, M. Ganzle, G. Vignolo, Development of gluten-free breads started with chia and flax seed sourdoughs fermented by selected lactic acid bacteria, 125 (LWT - Food Science and Technology, 2020), Article 109189
S. Schreyer, C. Klein, A. Pfeffer, J. Rasinska, L. Stahn, K. Knuth, B. Abuelnor, A.E.C. Panzel, A. Rex, S. Koch, S. Hemmati-Sadeghi, B. Steiner, Chia seeds as a potential cognitive booster in the APP23 Alzheimer’s disease model. Sci. Rep. 10, 18215 (2020)
M.E. Oliva, D. Rosario Ferreira, M. Joubert, M. B. V., & M.E. D’Alessandro (2021). Salvia hispanica L. (chia) seed promotes body fat depletion and modulates a dipocyte lipid handling in sucrose-rich diet-fed rats. Food Research International, 139, Article 109842
A.M. Abdel-Aty, A.M. Elsayed, H.A. Salah, R.I. Bassuiny, S.A. Mohamed, Egyptian chia seeds (Salvia hispanica L.) during germination: Upgrading of phenolic profile, antioxidant, antibacterial properties and relevant enzymes activities. Food Sci. Biotechnol. 30, 723–734 (2021)
Y.S. Velioglu, G. Mazza, L. Gao, B.D. Oomah, Antioxidant activity and total phenolics in selected fruits, vegetables, and grain products. J. Agricultural Food Chem. 46, 4113–4117 (1998)
K.H. Kim, R. Tsao, R. Yang, S.W. Cui, Phenolic acid profiles and antioxidant activities of wheat bran extracts and the effect of hydrolysis conditions. Food Chem. 95, 466–473 (2006)
C. Ao, A. Li, A.A. Elzaawely, T.D. Xuan, S. Tawata, Evaluation of antioxidant and antibacterial activities of Ficus microcarpa L.fil. extract. Food Control 19, 940–948 (2008)
R. Re, N. Pellegrini, A. Proteggente, A. Pannala, M. Yang, C. Rice-Evans, Antioxidant activity applying an improved ABTS radicalcation decolorization assay. Free Radic. Biol. Med. 26, 1231–1237 (1999)
G.L.( Miller, Use of dinitro-salicylic acid reagent for the determination of reducing sugar. Anal. Chem. 31, 426–429 (1959)
Y.Z. Gunata, C.L. Bayonove, R.E. Cordonnier, A. Arnaud, P. Galzy, Hydrolysis of grape monoterpenyl glycosides by Candida molischiana and Candida wicherhamii β-glucosidase. J. Sci. Food Agric. 50, 499–506 (1990)
S. Sirin, S.B. Aydaş, B. Aslım, Biochemical evaluation of phenylalanine ammonia lyase from endemic plant cyathobasis fruticulosa (Bunge) aellen. for the dietary treatment of phenylketonuria. Food Technol. Biotechnol. 54 B, 296–303 (2016)
A.W. Bauer, W.M. Kirby, J.C. Sherris, M. Turk, J. Am, Antibiotic susceptibility testing by a standardized single disk method. Clin. Pathol. 44, 493 (1996)
National Committee for Clinical Laboratory Standards, Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically, Approved Standard M7–A7, vol. 26 (Clinical and Laboratory Standards Institute, Wayne, PA, 2009)
N. Liu, M. Song, N. Wang, Y. Wang, R. Wang, X. An, J. Qi, The effects of solid-state fermentation on the content, composition and in vitro antioxidant activity of flavonoids from dandelion. PLOS ONE 15, e0239076 (2020)
H.M. Abdel-Mageed, A.Z. Barakat, R.I. Bassuiny, A.M. Elsayed, H.A. Salah, A.M. Abdel-Aty, S.A. Mohamed, Biotechnology approach using watermelon rind for optimization of α-amylase enzyme production from Trichoderma virens using response surface methodology under solid-state fermentation (Folia Microbiologica, 2021), https://doi.org/10.1007/s12223-021-00929-2
S.J. Hur, S.Y. Lee, Y.-C. Kim, I. Choi, G.-B. Kim, Effect of fermentation on the antioxidant activity in plant-based foods. Food Chem. 160, 346–356 (2014)
L.N. Hlahla, F.N. Mudau, I.K. Mariga, Effect of fermentation temperature and time on the chemical composition of bush tea (Athrixia phylicoides DC). J. Med. Plants Res. 4, 824–829 (2010)
R.K. Salar, M. Certik, V. Brezova, Modulation ofphenolic content and antioxidant activity of maize bysolid state fermentation with Thamnidium elegans CCF1456. Biotechnol. Bioprocess. Eng. 17, 109–116 (2012)
J. Moore, Z. Cheng, J. Hao, G. Guo, J.G. Liu, C. Lin, L.L. andYu, Effects of solid-state yeast treatment onthe antioxidant properties and protein and fiber compositions of common hard wheat bran. J. Agric. FoodChem 55, 10173–10182 (2007)
T. Bhanja, S. Rout, R. Banerjee, B.C. Bhattacharyya, Studies on the performance of a new bioreactor for improving antioxidant potential of rice. LWT-Food Sci. Technol. 41, 1459–1465 (2008)
T.M. Ðordevic, S.S. Siler-Marinkovic, S.I. Dimitrijevic-Brankovi, Effect of fermentation on antioxidant properties of some cereals and pseudo cereals. Food Chem. 119, 957–963 (2010)
S. Cai, O. Wang, W. Wu, S. Zhu, F. Zhou, B. Ji, F. Gao, D. Zhang, Comparative study of the effects of solid-state fermentation with three filamentous fungi on the total phenolics content (TPC), flavonoids, and antioxidant activities of sub-fractions from oats (Avena sativa L.). J. Agric. Food Chem. 60, 507–513 (2012)
T. Bhanja Dey, R.C. Kuhad, Upgrading the antioxidant potential of cereals by their fungal fermentation under solid-state cultivation conditions. LettAppl Microbiol. 59, 493–499 (2014)
N. Ritthibut, S. Oh, S. Lim, Enhancement of bioactivity of rice bran by solid-state fermentation with Aspergillus strains. LWT-Food Sci. Technol. 135, 110273 (2021)
I.-C. Sheih, T.J. Fang, T.-K. Wu, R.-Y. Chen, Effects of fermentation on antioxidant properties and phytochemical composition of soy germ. J. Sci. Food Agric94, 3163–3170 (2014)
Z. Xue, M. McCluskey, K. Cantera, A. Ben-Bassat, F.S. Sariaslani, L. Huang, Improved production of p-hydroxycinnamic acid from tyrosine using a novel thermostable phenylalanine/tyrosine ammonia lyase enzyme. Enzym Microb. Technol. 42, 58–64 (2007)
B. DeLange, D.J. Hyett, P.J.D. Maas, D. Mink, F.B.J. van Assema, N. Sereinig, A.H.M. de Vries, J.G. de Vries, Asymmetric synthesis of (S)-2-indolinecarboxylic acid by combining bio-catalysis and homogeneous catalysis. Chem. Cat Chem. 3, 289–292 (2011)
T. Bhanja, A. Kumari, R. Banerjee, Enrichment of phenolics and free radical scavenging property of wheatkoji prepared with two filamentous fungi. BioresourTechnol 100, 2861–2866 (2009)
Q. Shahzad, S. Javed, S. Mahmood, A. Hammed, Phenylalanine ammonia lyase (PAL) by Bacillus subtilis GCB- 31 on agro-industrial wastes. Int. J. Biosci. 15, 233–240 (2019)
L. Liu, W. Wen, R. Zhang, Z. Wei, Y. Deng, J. Xiao et al., Complex enzyme hydrolysis releases antioxidative phenolics from rice bran. Food Chem. 214, 1–8 (2017)
F. Ali, F. Rahul Naz, S. Jyoti, Y.H. Siddique, Health functionality of apigenin: A review. Int. J. Food Prop. 20, 1197–1238 (2017)
W.H. Salama, A.M. Abdel-Aty, A.S. Fahmy, Rosemary leaves extract: Anti-snake action against Egyptian Cerastes cerastes venom. J. Traditional Complement. Med. 8, 465–475 (2018)
A.M. Abdel-Aty, W.H. Salama, M.B. Hamed, A.S. Fahmy, S.A. Mohamed, Phenolic-antioxidant capacity of mango seed kernels: therapeutic effect against viper venoms. Revista Brasileira de Farmacognosia 28, 594–601 (2018)
A.Z. Barakat, A.R. Hamed, R.I. Bassuiny, A.M. Abdel-Aty, S.A. Mohamed, Date palm and saw palmetto seeds functional properties: Antioxidant, anti-inflammatory and antimicrobial activities. J. Food Meas. Charact. 14, 1064–1072 (2020)
M.C. MacDonald, P. Arivalagan, D.E. Barre, J.A. MacInnis, G.B. D’Cunha, Rhodoto rulaglutinis phenylalanine/tyrosine ammonia lyase enzyme catalyzed synthesis of the methyl ester of para-hydroxycinnamic acid and its potential antibacterial activity. Front. Microbiol. 7, 1–11 (2016)
J.H. Yang, Y.H. Tseng, Y.L. Lee, J.L. Mau, Antioxidant properties of methanolic extracts from monascal rice. LWT-Food Sci. Technol. 39, 740–747 (2006)
Y. Xiao, X. Rui, G. Xing, H. Wu, W. Li, X. Chen, Solid state fermentation with Cordyceps militarisSN-18 enhanced antioxidant capacity and DNA damage protective effect of oats (Avena sativa L.). J. Funct. Foods16, 58–73 (2015)
Y. Semaming, P. Pannengpetch, S.C. Chattipakorn, N.P. Chattipakorn. (2015) Pharmacological Properties of Protocatechuic Acid and Its Potential Roles as Complementary Medicine. Evidence-Based Complementary and Alternative Medicine, ID 593902
A.A.A. Mohdaly, A.A. Mahmoud, M.H.H. Roby, I. Smetanska, M.F. Ramadan, Phenolic extract from Propolis and bee polien: Composition, antioxidant and antibacterial activities. J. Food Biochem. 39, 538–547 (2015)
H. Taleb, S.E. Maddocks, R.K. Morris, A.D. Kanekanian, The Antibacterial Activity of Date Syrup Polyphenols against S. aureus and E. coli. Front. Microbiol. 7, 00198 (2016)
M. Matejczyk, R. Swisłocka, A. Golonko, W. Lewandowski, E. Hawrylik, Cytotoxic, genotoxic and antimicrobial activity of caffeic and rosmarinic acids and their lithium, sodium and potassium salts as potential anticancer compounds. Adv. Med. Sci. 63, 14–21 (2018)
S.A. Mohamed, R.M. Saleh, S.A. Kabli, S.M. Al-Garni, Influence of solid-state fermentation by Trichoderma spp. on solubility, phenolic content, antioxidant, and antimicrobial activities of commercial turmeric. Biosci. Biotechnol. Biochem. 80, 920–928 (2016)
H.A. Salah, R.I. Bassuiny, M.I. El–Khonezy, A.S. Fahmy, S.A. Mohamed, Impact of solid-state fermentation by Trichoderma spp. on phenolic content, antioxidant and antibacterial activities of curry leaf powder. J. Food Meas. Charact. 13, 1333–1340 (2019)
Acknowledgements
The authors are grateful to the National Research Centre (NRC) in Cairo, Egypt, for basically funding this study under contract No. 12020110.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Abdel-Aty, A.M., Barakat, A.Z., Bassuiny, R.I. et al. Improved production of antioxidant-phenolic compounds and certain fungal phenolic-associated enzymes under solid-state fermentation of chia seeds with Trichoderma reesei: response surface methodology-based optimization. Food Measure 16, 3488–3500 (2022). https://doi.org/10.1007/s11694-022-01447-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11694-022-01447-6