Abstract
In this study, Biarum bovei extract was used to produce bioactive peptides from wheat gluten protein and the biological and functional properties of the hydrolysates were determinated. The results showed that Biarum bovei extract has its highest protease activity (7.3 U/mg protein) at 45 °C and pH 5. Based on electrophoresis analysis, the molecular weight of hydrolysate was < 10 kDa. F1 fraction had the highest antioxidant activity in DPPH (65.85 ± 2.64 µmol TE/g)) and ABTS radical scavenging assays (295.81 µmol TE/g). F2 fraction with 86.3 ± 0.48 had the ability to inhibit the ACE enzyme. The F3 and F1 fractions had statistically the highest inhibition rate (49.37 ± 0.12%. and 79.19 ± 1.13%) in alpha-glucosidase and alpha amylase, respectively. The F1, F2 fractions hydrolysate had an inhibitory effect on Escherichia coli, Staphylococcus aureus, Listeria monocytogenes and Bacillus cereus. Functional properties of hydrolysates with increasing molecular weight, increased significantly. The presence of high levels (p ≤ 0.05) of amino acids with hydroxyl groups, hydrophobic and positive charged in fractions had critical role on biological and technological activity. These findings confirmed the efficiency of gluten hydrolysates with low molecular weight (F1 < 3 kDa) on biofunctionality such as scavenging radical activity, ACE inhibitory, antidiabetic and antibacterial activity could be beneficial from health and technological perspectives.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Amino acid residues (2–20, MW < 6 kDa) typically exist in bioactive peptides but, they are inactive. After hydrolysis of the parent protein by digestive or microbial enzymes, or during processing, they exert their physiological, biological, and functional effects. Depending on their structural characteristics (i.e., sequence of amino acids and composition), peptides can play a variety of roles, including; minerals binding, opioids, regulating immune activity, as antimicrobials and antioxidant or as lowering cholesterol and blood pressure agent [1]. The increase of the functional foods market can also be explained by other factors, e.g., the improved life quality of older people, the steady increase of life expectancy, and the healthcare cost [2, 3]. In addition, numerous peptides have been found to have multiple properties. So far, antioxidant activities of bioactive peptides and hydrolysates derived from the protein of sunflower [4], walnut kernel [5], beans [6], egg yolk [7], milk casein [8], shrimp [9], tilapia [10] and many other substances have been studied. The antimicrobial properties of peptides depend on their structural properties, molecular weight, amino acid composition, hydrophobicity, and secondary structure. Researchers reported that peptides extracted from the addition of probiotics to cottage cheese reduced the Listeria monocytogenes within the 20-days storage [11]. The peptides which can lowering blood pressure are often short-chain (2–12 amino acids) containing acidic amino acids such as aspartic acid and glutamic acid, in a means time they are positively charged (containing an alkali group at the amino terminus), and mostly hydrophobic [12]. Many studies have been reported on rice [13], soy [14], peas [15], and flaxseed hydrolysates [16] with diverse bio-functionality.
Biarum bovei (Kardeh) with the scientific name of Biarum carduchcorum. It is an edible, delicate plant with broad leaves, three species of which grow in Iran [17]. Researchers reported that by adding the aqueous extract of Biarum bovei has 100–150 units of the enzyme in each kilogram of the beef, examined the characteristics of kielbasa produced. Although the aqueous extract of the Biarum bovei caused the meat to be crispy, increased the emulsifying ability, and significant improvement in the relevant indicators, the use of crushed meat did not improve the texture consistency of the produced kielbasa and had no significant effect on its sensory acceptance [13, 17].
Wheat gluten is an inexpensive protein source compared with other vegetable proteins having high thiol groups and excellent functional properties. It can be classified into two main sub-groups: monomeric gliadins (α-, β-, γ- and ω-gliadins) and polymeric glutenins (high molecular weight, HMW, and low molecular weight, LMW, glutenins) [18]. In recent years, the production of bioactive peptides from food by-products has been considered. The use of these inexpensive compounds due to the reduction of production costs, increased added value, and efficient consumption of waste.
Our hypothesis is that the Biarum bovei extract can produce different peptides from wheat gluten with diverse bio-functionality. Therefore, this study aimed to examine the impact of partially purified Biarum bovei enzyme on the hydrolysis of wheat gluten (by-product of starch industry) to obtain the potentially bio-active peptides. Several molecular weight cut-offs (MWCOs) of ultrafiltration (UF) membranes were used to sieve the produced peptides as well as the assessment of gluten degradation by SDS-PAGE. Functional properties (i.e., solubility, emulsion properties and foam characterization) of peptides were determined and then biological properties of the peptides such as antioxidant activity (DPPH• and ABTS•), ACE inhibitory, antibacterial activity as well as antidiabetic properties were elucidated and finally the amino acid compositions of the hydrolysates were also determined. The findings of this study not only opens new horizons on the application of starch industry by-products (i.e., wheat protein) but it could also provide new insights into the utilization of gluten hydrolysates as bio-functionality agents.
Materials and methods
Wheat gluten (80.45% protein) was purchased from the Shahdineh factory (Esfahan, Iran). The approved (Department of Botany, Shiraz University, Iran) variety of Biarum bovei were collected (May to June, 2020) from local farms (Kazeron city, Fars province, Iran). All chemicals used in this study were provided by Merck and were of analytical grade.
Preparation of enzymatic extract
After Biarum bovei collection, the samples were washed with deionized water and dried in the laboratory (25 °C), then ground by an electric grinder (Honda 8 hp OHV engine, USA) and stored in the refrigerator. 100 g of Biarum bovei were mixed with 600 ml of sodium chloride solution (0.85%), stirring (24 h), filtered and then centrifuged and the supernatant was kept at 4 °C until further use. The crude enzyme, which was extracted in the previous step, was mixed with ammonium sulfate (65% saturation) to precipitate the protease. To do this, ammonium sulfate was first added to the sample in the presence of ice and dissolved well (65% saturation) and allowed to stir (3 h, 4 °C), then centrifuged (12,000×g, 15 min, 4 °C) and the precipitate was dialyzed (cut off 14,000 Daltons) against phosphate buffer (pH 7, 4 °C) for 24 h [19].
Determination of enzymatic activity
Protease activity of the Biarum bovei extract was tested at room temperature in phosphate buffer (50 mM containing 5 mM DTT, 2 mM EDTA, and 5 mM L-cysteine, pH 6.5) using casein as substrate. First, the enzyme extract (50 μl) was mixed with buffer (350 μl), then casein (400 μl of 1% (w/v)) was added and the reaction was performed (35 °C for 60 min) at 10 min intervals. The reaction was then stopped by trichloroacetic acid solution (10%, 800 μl). The sample was kept (30 min) at room temperature, centrifuged (12,000×g, 10 min), and the absorbance of supernatant was read (λ = 280 nm). An enzyme unit means the amount of enzyme that can convert preotein (e.g., casein) to the equivalent products (e.g., tyrosine amino acid). Various concentrations (0.1–1 mM) of tyrosine amino acid were also used to plot the standard curve [20]. The quantity of soluble protein was determined by using the Bradford procedure at 595 nm with bovine serum albumin (BSA, 0.1–1 mg/ml) as a standard protein. All experiments related to activity were done with three replications.
The hydrolysis process and separation of peptide via ultrafiltration
The hydrolysis process of wheat proteins was performed using the Biarum bovei extract (pH 5, 45 °C). In a reaction container equipped with a stirrer, wheat glutens (WG) powder was dispersed (5% w/v) in distilled water (85 °C for 10 min), before adding the enzyme, the pH and temperature were regulated. Enzyme with a 1:10 substrate: enzyme ratio (based on WG protein content) was added to the dispersion and the enzymatic digestion process was performed (6 h, under the same constant conditions). The pH of the mixture was fixed by the pH-State method (NaOH = 1 N). Upon the completion of the digestion, the excess enzyme was inactivated by immersing the reaction chamber in a 90 °C water bath for 10 min, then immediately cooled down in ice pool. The insoluble components were allowed to sediment (8000 g, 4 °C, 15 min), the supernatant filtered and vacuum dried, then the obtained powder was stored in the refrigerator until further processing and the degree of hydrolysis was calculated by the pH–stat method [13, 21]. Wheat gluten hydrolysate (WGH) was then filtered through 100, 30 and 3 kDa and finally, fractions 4 were isolated.
SDS–PAGE electrophoresis
The gels were prepared with a 5% stacking gel (pH 6.8) and 15% polyacrylamide resolving gel (pH 8.8). Until loading the samples onto the gels, they were dissolved in sample buffer (0.1 M Tris–HCL, pH6.8, 2% SDS, 5% -mercaptoethanol, and 0.02 percent bromophenol blue). Using methanol (40% v/v) and an acetic acid (7% v/v) solution, the gels were stained with Comassie brilliant blue R-250 (0.125% w/v), then de-stained in the same solution [22].
Determination of antioxidant properties
DPPH free radical scavenging activity
The free radical scavenging activity of 2,2-diphenyl-1-picrylhydrazyl was measured using the method of Wang [23] with slight modification. For this, 500 μl of DPPH solution (0.16 mM in 96% ethanol) was mixed with 500 μl of the sample in a 1.5 ml microtube. The control sample was prepared by replacing the sample with distilled water. The mixture was then vortexed well and kept at room temperature in the dark for 30 min. After this period, the absorbance of the samples in a 96-cell plate and at a wavelength of 517 nm was read by spectrophotometer (Agilent-Carry 60, California, USA). The ability to inhibit DPPH radicals was expressed using the standard Trolox curve (10–200 μmol/ml) per mg of dry matter.
ABTS free radical scavenging activity
The method of Gimenez [24] was used to evaluate the ability of ABTS radical inhibition. 7 mM ABTS solution in 2.45 mM potassium persulfate was prepared in a dark glass container and kept at room temperature in a dark place for 16 h. After that, dilution was performed with distilled water to reach an absorption of 0.7 at 764 nm. Then 20 μl of the sample was mixed with 980 μl of diluted ABTS solution and kept in a dark place at 30 °C for 10 min, after which the adsorption time of the samples was read at 734 nm. The ability to inhibit ABTS cation radicals was calculated using the standard Trolox curve formula (50–1100 μmol/ml).
Angiotensin I converting enzyme inhibitory activity
50 μl of the sample (hydrolyzed protein and each of the peptide fractions) was mixed with 50 μl of ACE solution (25 units/ml) and incubated at 3 °C for 5 min. The resulting mixture was then incubated with 150 μl of the substrate ((Hip-His-Leu), 8.3 mM in 50 mM sodium borate buffer) for 60 min at the same temperature. The reaction was stopped by adding 250 μl of 1 M hydrochloric acid solution. After centrifugation at 3000 rpm for 15 min, 0.2 ml of the supernatant was transferred into the test tube and dried at 80 °C for 1 h. Hypoboric acid was dissolved in 0.5 ml of distilled water and the absorption of the final solution was determined at 228 nm using a spectrophotometer. The IC50 index was also reported as the concentration required to inhibit 50% of the ACE enzyme [25].
Evaluation of the antibacterial activity
The micro-dilution method was used to determine the antibacterial properties of bioactive peptides derived from wheat gluten protein. For this purpose, after inoculation and activation of each of the pathogenic bacteria in Müller-Hinton broth culture medium at 37 °C for 24 h, a microbial suspension with a concentration of 104 CFU/ml was prepared. Then, different dilutions of the antibacterial agent (bioactive peptide) with a coefficient of one half were prepared and with a constant concentration (104 CFU/ml) of each of the studied bacteria and incubated in a 96-well microplate at 37 °C for 24 h. The first row of the plate was considered as negative control and only contained culture medium and antimicrobial compound with a certain concentration. The last row as a positive control contained only culture medium and bacterial suspension with a concentration of (104 CFU/ml). The final volume of each microplate well was considered to be 200 µl. After the incubation period, the light absorption of each well was determined with an ELISA reader at a wavelength of 600 nm and the least concentration of antibacterial agent (bioactive peptide) that inhibited the growth of each bacterium and had a turbidity of lower than positive control was considered as the minimum inhibitory concentration (MIC) [22, 26].
Anti-diabetic activity
Alpha-glucosidase enzyme inhibitory
Mouse intestinal alpha-glucosidase inhibitory activity of Connelly [27] was performed with slight modifications. Initially, a concentration of 20 mg/ml from the sample was made and then the enzyme was extracted from mouse intestinal powder by buffer and then the extract was diluted to 90 Mu/ml. Enzyme activity was measured at 37 °C and 405 nm for each sample. 5 mg/ml acarbose solution was used as a positive control.
Porcine alpha-amylase inhibition assay
The inhibitory activity of gluten enzymatic hydrolysates to the porcine alpha-amylase enzyme was evaluated by Connolly [27] with some modifications. First, a mixture of 100 μl of the enzyme solution (3.75 U/ml) with 100 μl of the sample was incubate at 37 °C for 10 min and then 200 μl of the starch solution (0.5% w/v) was added. The reaction was started and after 15 min at 37 °C, the reaction was stopped by adding 400 μl of DNS solution. Then immersed in boiling water for 5 min and immediately put on ice and diluted with 3 ml of distilled water. Finally, the absorbance of the mixture was read at 540 nm. Acarbose was also used as a positive control.
Functional properties
Solubility
The solubility of gluten hydrolysates at pH 2, 4, 7, 10 was evaluated by dissolving 100 mg of the hydrolysates and the control sample in 10 ml of distilled water, and 1 M NaOH or HCl were used to adjust the pH. The obtained solution was kept at room temperature for 30 min and centrifuged at 100 g at room temperature for 15 min. Then, the amount of floating surface protein was obtained by the Lowry method using the BSA [28].
Emulsifying properties
The Emulsifying Activity Index (EAI) and the Emulsion Stability Index (ESI) were performed using the Pearse [29] method with slight modifications. Sunflower oil was added at a rate of 10 ml to 30 ml of hydrolysate solution at a concentration of 2 mg/ml and after mixing, the pH of the samples was adjusted at 7. The mixture was homogenized at 12,000×g for 1 min. 50 μl of the formed emulsion was diluted 100 times using 0.1% sodium dodecyl sulfate solution. Then, the absorption rate of the sample was read at a wavelength of 500 nm. The same steps were repeated for the formed emulsion after 10 min at room temperature and the adsorption rate was obtained at the desired wavelength.
Foaming characteristics
The foaming expansion (FE) and foam stability (FS) of the produced from the hydrolysates were determined using the method of Shahidi [9] with slight modifications. 20 ml of hydrolysate solution at a concentration of 1% w/v was homogenized in a homogenizer for one minute at 12,000 rpm and room temperature. The obtained samples were transferred into a graduated cylinder and kept at room temperature for 10 min. The foaming expansion is the ability to expand the foam in zero minutes and the foam stability is the amount of foam diffusion after 10 min.
Amino acid analysis
The amino acid composition of the hydrolysates was determined using an automatic amino acid analyzer (Hitachi High-Technologies Co., Japan, L-8900) equipped with a Hitachi ion exchange resin column (60 mm × 4.6 mm id, 1 µm). Twenty amino acids from the sample powder were analyzed. The amino acid contents of hydrolysates were expressed as percent.
Statistical analysis
Every experiment described here was carried out independently in triplicate and the findings were recorded. Statistical analysis was calculated using analysis of variance with Duncan's analysis (5% level). The mean and standard deviation were calculated in Excel software. SPSS software version 18 was used to analyze the data and Excel software was used to draw the graphs.
Results and discussion
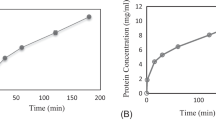
According to the findings, the protein content of the aqueous extract of Biarum bovei was 5.41 mg/ml. As can be seen in Fig. 1, the highest activity of the enzyme was at pH 5, which the enzyme activity was 7.3 (U/mg protein) and by increasing the pH to 9, enzyme activity decreased (2.19 U/mg protein) significantly (p ˂ 0.05). This indicates that the protease enzyme in Biarum bovei extract is acidic and its activity decreases in alkaline conditions so that the lower the pH of the extraction buffer and the closer to the optimum pH of activity, the higher the residual activity. In this respect, the enzymatic extract of the plant is similar to other aspartic proteases [30].
According to Fig. 1, with increasing temperature from 25 to 45 °C, a significant increase in the Biarum bovei enzyme activity was observed, which became flat with further increase to 55 °C. Therefore, enzyme activity of extract 5.76 (U/mg protein) at 45 °C was used as the most optimum activity temperature for hydrolysis. In a study, the maximum temperature for the enzymatic activity of aspartic enzyme extracted from Rhizopus oryzae was reported at 60 °C and stated that this enzyme is inactivated rapidly at high temperatures [31]. Similar results were observed by protease isolated from Biarum bovei which 45 °C and pH 5 were reported as the most suitable conditions for Biarum bovei extract activity and use of it to produce cheese from milk [17, 32].
SDS-PAGE analysis
By using partially purified Biarum bovei extract, the fractionated of the wheat gluten were hydrolyzed (6 h, 45 °C) and their SDS-PAGE analyses were performed in order to investigate the cleaving actions of Biarum bovei protease (Fig. 2). The electrophoretic pattern of gluten and hydrolysates confirms that the Biarum bovei enzyme extract was able to hydrolyze gluten. The effective degradation was occurred by enzyme and the high molecular weight-related bands (≥ 10 kDa) were disappeared (Fig. 2). The peptide profile of gluten which were digested during this study was similar to the one which was digested with pepsin and kiwifruit extract [10, 11]. These peptides are expected to express desirable functional properties namely; antioxidant properties, antihypertensive, and anti-diabetic properties [17]. Jayawardana [33] also showed that gluten hydrolysate fractions (< 3 kDa) had the highest alpha-amylase inhibitory and antioxidant activity.
Antioxidant properties
The DPPH radical scavenging activity of each component of the ultrafiltration membrane of gluten protein hydrolysates is shown in Table 1. The highest level of inhibition was observed in hydrolyzed components of less than 3 kDa and was 65.85 ± 2.64 µmol TE/g. The lowest level of inhibition was related to the component of > 100 kDa and was 18.25 ± 1.74 µmol TE/g. The researchers reported that smaller-sized peptides had a higher radical scavenging activity than the others ultrafiltration membrane components. Also, the type of amino acid in these hydrolysates would affect DPPH scavenging activity [14, 34].
Table 1 shows the ABTS radical scavenging activity of each ultrafiltration membrane component. The results showed that all components have ABTS radical scavenging activity and the highest scavenging rate is related to the component less than 3 kDa that is 295.81/2.5 µ molTE/g and the lowest scavenging activity was related to the component < 100 kDa that was 115.24 ± 5.48 µmol TE/g. These results were in good agreement with the research of Zhuang [35]. The researchers reported that a < 10 kDa component of corn hydrolyzed had more ABTS cation radical scavenging activity than a component with a higher molecular weight. Researchers have also reported that peptides with a molecular weight of less than 3 kDa from hydrolyzed pinto beans have a higher scavenging activity than other components with a higher molecular weight [7, 19]. Both F1 and F2 feactions are antioxidants, they contain positively charged (His and Arg) and hydrophobic (Pro) amino acids. On the other hand, Phe and Arg (Table 4) contents of F1 fraction was high. Based on F1 fraction amino acid composition results (Table 4), the high content of hydrophobic amino acids namely; Tyr (3.69%), Pro (14.47%), Val (6.92%), and Ala (6.75%) may be associated with antioxidant activity. The researchers stated that a possible explanation for these findings may be due to the abundance of hydrophobic and hydrophilic amino acids in peptides enriched during ultrafiltration, especially at low molecular weights [14]. The antioxidant properties of low molecular weight peptides have been confirmed that due to their molecular weight because they can easily react with lipid radicals and reduce lipid peroxidation [36]. Like DPPH radicals, the molecular weight and structure of peptides and their amino acid sequence affect the rate of ABTS cation radical scavenging activity. Degradation of the natural structure of proteins by enzymatic hydrolysis leads to the denaturation and exposure of active amino acid groups that react with free radicals. These amino acids can act as electron donors in the presence of free electrons, thereby causing radical chain reactions by inhibiting the production of non-radical products and making antioxidant effects [43]. The hydrophobicity of peptides also plays an important role in their antioxidant activity, especially in inhibiting free radicals [37].
ACE blocking capability
The relative ACE inhibitory activity in all produced fractions is given in Table 1. Of the 4 fractions produced, 2 fractions, F2 (peptides with a molecular weight between 3 and 30 kDa) and F3 (peptides with a molecular weight between 30 and 100 kDa) have higher inhibitory activity than the others. The fraction F2 with 86.3 ± 0.48% had the highest inhibitory activity and then F3 with 81.3 ± 3.11% had the ability to inhibit this ACE enzyme (there was no significant difference between F2 and F3). The fraction F1 with 24.4 ± 0.55% has the least inhibitory capability. The high content of hydrophobic amino acids namely (Table 4); Tyr (3.69%), Pro (14.47%), Val (6.92%), and Ala (6.75%) may be associated with ACE blocking capability. From these results, it can be stated that the resulting peptides in two fractions F2 and F3 have the ability to bind to the active site of the ACE enzyme by hydrogen, hydrophobicity, and Van der Waals binding and reduce the activity of this enzyme by different mechanisms. The results obtained in comparison with the results of other researchers show that hydrolysates derived from gluten protein using Biarum bovei extracts have a better ability to reduce blood pressure. Cheung [38] reported that the hydrolysis of barley protein using the enzyme thermolysin was about 90%.
Antimicrobial ability
The minimum inhibitory concentrations of bioactive peptides produced by Biarum bovei extract on wheat gluten protein against Escherichia coli, Staphylococcus aureus, Listeria monocytogenes, and Bacillus cereus are shown in Table 2. According to the results, gluten protein (control sample) had no inhibitory effect on any of the bacteria, while F1 hydrolysates (with a molecular weight of less than 3 kDa) had inhibitory power on all bacteria. Hydrolysates of fraction F2 (peptides with a molecular weight between 3 and 30 kDa) also had inhibitory effect on Escherichia coli, Listeria, Bacillus cereus and Staphylococcus, but in higher concentrations than F1. Peptides of fraction F3 (hydrolysates with a molecular weight between 30 and 100 kDa) had only inhibitory effect on Escherichia coli and Listeria monocytogenes and Bacillus, and hydrolyzers of fraction F4 (hydrolysates with a molecular weight greater than 100 kDa) had no inhibitory effect on any bacteria. The peptides did not show different activity Quantitatively and the results showed that Staphylococcus aureus was the most resistant bacterium to wheat gluten peptides and Listeria monocytogenes was the most sensitive bacterium to these peptides produced from Biarum bovei. Most peptides can exert their microbial effect directly by creating pores in the membrane and interfering with the passage of ions and nutrients. Molecular mechanisms and membrane permeation of different peptides may be affected by parameters such as amino acid sequence, membrane lipid composition, and peptide concentration [39]. Antibacterial peptides are usually composed of less than 50 (12–50) amino acids, have a molecular weight of 5–10 kDa and have cationic and amphipathic properties. Despite the different structures, the presence of a positive charge in many peptides enables them to bind to bacterial membranes [17, 26]. The high content of positive charge amino acids (Table 4) namely; His, Lys and Arg may be associated with ACE blocking capability. Antimicrobial peptides interact with the negatively charged parts of the bacterial cell membrane through their positively charged portions and thus accumulates on the surface of the target cell membrane. The degree of surface hydrophobicity of peptides also plays an important role in the development of their antibacterial properties, because the hydrophobic parts are responsible for communicating with hydrophobic components in the membrane. Through this interaction with the cell membrane, major rearrangements occur in the structure that may be the result of peptide-lipid binding, peptide transport along the cell membrane, and interaction with intracellular targets [40]. On the other hand, the antibacterial potential of hydrolyzed proteins increases to a certain threshold with an increasing degree of surface hydrophobicity, and then a further increase in surface hydrophobicity due to dimerization of proteins in aqueous solution reduces the antibacterial effect of hydrolyzed protein. Because protein dimers are able to pass through the cell membrane to react with the target membrane [41]. Pritchard [42] evaluated the antimicrobial activity of the bioactive peptides derived from cheddar cheese on bacteria (Escherichia coli, Staphylococcus aureus, and Bacillus cereus) using the micro-dilution method and showed that peptides with a molecular weight of less than 10 kDa had the greatest antimicrobial effect on Bacillus cereus and the least inhibitory effect on Staphylococcus aureus, which is consistent with the results of the present study.
Inhibition asssay
The results showed that all the fractions of hydrolysates produced in the concentration used had the inhibitory effect of the alpha-glucosidase enzyme in the rat intestine (Table 3). The inhibitory levels of all four hydrolysates were significantly different from each other. It showed the highest inhibition rate in the hydrolysates between 30 and 100 kDa (F3) with a value of 49.37 ± 0.12%. Fraction inhibitory capacity greater than 100 kDa (F4) was the lowest at 25.15 ± 0.33 and did not show a significant difference with F1 (24.70 ± 0.11) and F2 (25.44 ± 0.26) hydrolysates. The specificity of this enzyme and the presence of hydroxylic and hydrophobic amino acids in this peptide may be the reason for the higher inhibitory capacity of this fraction compared to other fractions. The high content of hydrophobic amino acids namely; Tyr (3.69%), Pro (14.47%), Val (6.92%), and Ala (6.75%) may be associated with inhibitory capability. Tests of the structural activity patterns shows that alpha-glucosidase and inhibitory peptides interact more through hydrogen bonds and electrostatic tendencies [22, 27]. The high inhibitory activity of this peptide could be attributed to its high Pro content. This is in line with previous reports in which amino acids act as inhibitors of α-glucosidase. Pro, Gly, and Tyr derived from sardine muscle hydrolysate were reported as the most active compounds against diabetes [24]. The results showed that all hydrolysates had the ability to inhibit amylase. In the produced fractions, the fraction F1 (less than 3 kDa) with 79.19 ± 1.13% had the highest inhibition and the fraction F4 (greater than 100 kDa) had the lowest inhibition rate of 40.07 ± 2.92. The inhibition rate of fractions F2 and F3 were measured to be 57.83 ± 2.33% and 44.25 ± 0.95, respectively. Ngoh [36] reported that a fraction smaller than 2 kDa has the greatest inhibitory effect on alpha-amylase. The proposed mechanism for the activity of this enzyme is a multiple attack mechanism and it is a sliding movement through which the enzyme moves without separating from the precursor and releases the sugar units. The interaction between starch and enzyme is relatively weak and occurs through hydrogen and van der Waals bonds. It has been suggested that the peptides identified in this study contained proline amino acids at the amino and carboxylic ends [43]. Neves [37] reported that the ability of black bean protein to be antiseptic and anti-diabetic is due to the presence of aspartic acid, glutamic acid, valine, and serine.
Functional properties
Solubility is considered as one of the most important properties of proteins and hydrates due to the use of these compounds in food products, pharmaceuticals and medicine, and other industries [44]. Figure 3 shows the solubility of hydrolyzed gluten (F4, F3, F2, F1, gluten) at pHs 7, 4, 2, and 10. The solubility of standard bovine serum albumin was 96, 98, 99, and 98% for the pHs 2, 4, 7, and 10, respectively. At pH 2, the solubility was low in all samples, indicating the low solubility of these hydrolysates in acidic environments. While the standard sample (BSA) had good solubility at this pH. At pH 4 and 7, with increasing molecular weight, the solubility of the samples increased significantly, and in alkaline environments, the decrease in acidity had a positive effect on the solubility of the hydrolysates. They also reported that hydrolysates with smaller molecular weights tend to have more hydrogen and electrostatic bonds due to their polarity and have higher solubility [34]. The hydrolysis of sole fish gelatin [45] is consistent with the fact that in all these studies, the solubility increases with increasing pH from 5 to 7.
There are significant differences (p ˂ 0.05) between samples and control in emulsifying activity (Fig. 3). The highest emulsifying ability is related to the standard sample (BSA) (160 m2/g) followed by F1 (hydrolysates less than 3 kDa; 142 m2/g) and then F2 (hydrolysates with a molecular weight between 3 and 30 kDa; 111 m2/g), indicating that the emulsifying power of the sample decreases with an increasing molecular weight of the sample. The high content of hydrophobic amino acids namely; Tyr (3.69%), Pro (14.47%), Val (6.92%), and Ala (6.75%) may be associated with emulsion properties. The results are consistent with the results of Hajfathalian [46], in which it was reported that as the molecular weight decreases, the rate of hydrophobicity increases, which will lead to an increase in EAI probably due to a slight increase in the number of small and healthy fat cells and lead to unfolding and rearrangement of the molecule in the emulsion intermediate and leads to an increase in the oil-in-water levels and ultimately increase the ability to form the emulsion. The emulsion stability in the standard sample was higher than other hydrolysates (160 min) (Fig. 3). The stability of the emulsion in the control sample was 90 min and it significantly increased in other samples with increasing molecular weight. Low molecular weight molecules and proteins, due to their lower outer surface than heavier proteins, cannot well create a viscous environment and this will reduce the emulsion stability [47]. Smaller molecules are less efficient at reducing interfacial stress and hence the emulsion of these compounds will break down at a higher rate. Alolod [48] stated that the emulsion properties of gelatin hydrolysates of unicorn skin depend to a large extent on the concentration and size of peptides. Smaller peptides move faster into the water–oil interfacial space and form an emulsion, but emulsions are not very stable due to the reduced hydrophobic-hydrophilic balance on both sides of the emulsion surfaces.
The highest foaming capacity is for the standard sample (bovine serum albumin) followed by the control sample (hydrolyzed gluten) (95%), F4 fraction (93%), F3 fraction (78%), F2 fraction (52%), and the lowest value is related to F1 fraction (42%) (Fig. 3). Alolod [48] reported that the foaming capacity of the hydrolysates of unicorn fish skin gelatin was 5.83% higher than the control sample. In this study, the foaming capacity depended on the molecular weight of the hydrolysates, and the weakness in the foaming capacity was observed in low molecular weight hydrolysates. High molecular weight hydrolysates (F3, F4 fractions) and the control sample did not need to create force to form the foam structure due to their ability to regenerate the surface between air and water [46]. The results of foam stability after 10 min showed foam stability in control samples and hydrolysates with higher molecular weight. The stability of the formed foam was significantly different among all samples (p ˂ 0.05). In the control sample, the rate was 93%, which reached 85% in the F4 sample and also decreased to 60% in the F3 sample. A similar result was also reported by Alolod [48]. Decreasing foam stability in low molecular weight hydrolyzed samples due to less protein–protein interaction will not be able to form a suitable film and layer to prevent air from escaping from the foam cells and this will reduce the foam stability [49].
Amino acid composition
Table 4 represents the amino acid composition of wheat gluten hydrolysates. Essential and non-essential amino acids in hydrolysates were in the range of (25.08–28.39%) and (68.22–73.84), respectively. There is an adequate amount of both essential and non-essential amino acids, however, the essential amino acid content of the samples was higher than that recommended by FAO/WHO for children (23.2%) [14]. It also contains high amount of glutamic acid (2.01–5.46%), glycine (2.51–6.95%) and arginine (1.34–3.68%) which are vital for many bodily functions. Glutamic acid and glycine play a critical role in memory, learning and neurotransmitter. A key role for arginine is in blood circulation, wound healing, erectile dysfunction treatment, and immune function [4, 35]. The amino acids associated with biological activity in F3 registered 59.60% of hydrophobic, 5.85% of amino acids with a positive charge, 7.79% of aromatic amino acids. The major amino acids found in hydrolysates were glutamic acid (Glu), glycine (Gly), aspartic acid (Asp), proline (Pro), alanine (Ala), Cysteine (Cys), Glutamine (Gln), and Valine (Val). All the gluten hydrolysates were found to be rich in proline (12.44–14.47%) and glutamine (22.75–29.97%). There were differences (p < 0.05) in the amino acid composition between the hydrolysates, which could be the reason why the biological activity of F1 was slightly better as described in sections. The COVID-19 pandemic has widely affected the food sector with consumers increasingly seeking sustainable, organic, and functional foods [50]. The COVID-19 pandemic generated opportunities and challenges for the commercialization of innovative functional foods and nutraceuticals containing target bioactive compounds (e.g., Vitamins and antioxidants) and highlighted the advances of personalized nutrition to boost consumers immune system and improve their overall health [51]. These bioactive ingredients can boost enough our immune system to prevent or cure COVID-19. Nevertheless, their ability to boost the human immune system highlights their prospect of use in functional foods and presence in the nutraceuticals market [52].
Conclusion
The observations of this study suggest that gluten protein, starch industry by-product, is a talented source of biofunctionality peptides. Functional properties of hydrolysates with increasing molecular weight, increased significantly. Radical scavenging activity, the ability to inhibit the ACE enzyme and antibacterial capability belonged to F1 fraction of hydrolysate. The F1 and F3 fractions fractions had the highest inhibition rate in alpha-glucosidase and alpha amylase. The presence of high levels of amino acids with hydrophobic and positive charged in fractions had critical role on biological and technological activity. Considering these biofunctionality, these peptides have the potential to be used as a functional food ingredient.
Abbreviations
- MW:
-
Molecular weight
- WG:
-
Wheat gluten
- WGH:
-
Wheat gluten hydrolysate
- DH:
-
Degree of hydrolysis
- ACE:
-
Angiotensin I converting enzyme
- MIC:
-
Minimum inhibitory concentration
- F1:
-
Peptides with a molecular weight ≤ 3 kDa
- F2:
-
Peptides with a molecular weight 3–30 kDa
- F3:
-
Peptides with a molecular weight 30–100 kDa
- F4:
-
Peptides with a molecular weight ≥ 100 kDa
References
R. FitzGerald, M. Dermiki, Physicochemical and gelling properties of whey proteinhydrolysates generated at 5 and 50 °C using Alcalase and Neutrase, effect of total solids and incubation time. Int Dairy J 110, 104792 (2020)
C.M. Galanakis, The functionality of Food Components and Emerging Technologies. Foods 10, 128 (2021)
M.C. Galanakis, Separation of functional macromolecules and micromolecules: From ultrafiltration to the border of nanofiltration. Trends Food Sci. Technol. 42, 44–63 (2015)
C.M. Montone, A.L. Capriotti, C. Cavaliere, G. La Barbera, S. Piovesana, R.Z. Chiozzi, A. Laganà, Characterization of antioxidant and angiotensin-converting enzyme inhibitory peptides derived from cauliflower by-products by multidimensional liquid chromatography and bioinformatics. J. Funct. Foods 44, 40–47 (2018)
L. Feng, X. Wang, F. Peng, J. Liao, Y. Nai, H. Lei, M. Li, H. Xu, Walnut protein hydrolysates: play a protective role on neurotoxicity induced by d-galactose and Aluminum chloride in mice. Molecules 23, 2308 (2018)
L. Chen, E. Eckert, J. Han, K. Swallow, Z. Tian, M. Parra, Effects of enzymatic hydrolysis and ultrafiltration on physicochemical and functional properties of faba bean protein. Cereal Chem. 96(4), 15 (2019)
Y. Su, Y. Gao, J. Li, C. Chang, C. Wang, Y. Yang, Effect of enzymatic hydrolysis on heat stability and emulsifying properties of egg yolk. Food Hydrocoll. 97, 105224 (2019)
M. Diaz, E.A. Decker, Antioxidant mechanisms of caseinophosphopeptides and casein hydrolysates and their application in ground beef. J. Agric. Food Chem. 52(26), 8208–8213 (2004)
F. Shahidi, P. Ambigaipalan, Bioactive peptides from shrimp shell processing discards: antioxidant and biological activities. J. Funct. Foods. 34, 7–17 (2017)
D.M. Bernardi, L.D. Deparis, F. Dieterich, F.G.D. Silva, W.R. Boscolo, Production of hydrolysate from processed Nile tilapia (Oreochromis niloticus) residues and assessment of its antioxidant activity. Food Sci. Technol. 34(6), 709–716 (2016)
M.L. Timón, A.I. Andrés, J. Otte, M.J. Petrón, Antioxidant peptides (<3 kDa) identified on hard cow milk cheese with rennet from different origin. Food Res. Int. 120, 643–649 (2019)
S. Piovesana, A.L. Capriotti, C. Cavaliere, Recent trends and analytical challenges in plant bioactive peptide separation, identification and validation. Anal. Bioanal. Chem. 410, 3425–3444 (2018)
C. Moritani, K. Kawakami, A. Fujita, H. Shimoda, T. Hatanka, S. Tsuboi, Isolation of activating factors of serotonin N-acetyltransferase from rice peptides. J. Funct. Foods. 41, 148–154 (2018)
J. Wu, X. Ding, Characterization of inhibition and stability of soy-protein-derived angiotensin I-converting enzyme inhibitory peptides. Food Res. Int. 35(4), 367–375 (2002)
R.E. Aluko, T.L. Pownall, C. Udenigwe, amino acid composition and antioxidant properties of pea seed (Pisum sativum L.) enzymatic protein hydrolysate fractions. J. Agric. Food Chem. 58(8), 4712–4718 (2010)
P.J. Shand, P.W. Marambe, J.P.D. Wanasundara, An In-vitro Investigation of Selected Biologic Activities of Hydrolysed Flaxseed (Linum usitatissimum L.) Proteins. Am. Oil Chem. Soc. Publ. 85, 1155–1164 (2008)
S.S. Shekarforoush, M. Raeisi, M. Aminlari, H.R. Gheisari, H. Golkari, Study on physico- chemical properties of emulsion type sausage produced with aqueous extract of Biarum carduchcorum tenderizied meat. J. Food Hygiene 7, 26 (2017)
B. Mousavi, M. Kadivar, Effect of brine solution as a wheat conditioner, on lipase, amylase, andlipoxygenase activities in flour and its corresponding dough rheological properties. J. Food Process. Preserv. 42(6), 13631 (2018)
B. Wang, C.G. Yu, H.Y. Luo, Y.L. Qu, L.Y. Yang, Studies on the preparation and antioxidant properties of enzymatic hydrolysate from Dasyatis akajei by papain. Food Sci. Technol. Int. 10, 113–118 (2010)
A. Homaei, R. Etemadipour, Improving the activity and stability of actinidin by immobilization on gold nanorods. Int. J. Biol. Macromol. 72, 1176–1181 (2015)
K. Rezaei, F. Alavi, M. Jamshidian, Applying native proteases from melon to hydrolyze kilka fish proteins (Clupeonella cultriventris caspia) compared to commercial enzyme Alcalase. Food Chem. 14, 314–322 (2019)
U.K. Laemmli, Cleavage of structural proteins during assembly of head of bacteriophage-T4. Nature 227, 680–685 (1970)
W. Wang, Q.J. Liu, H. Cui, Rapid desalting and protein recovery with phenol after ammonium sulfate fractionation. Electrophoresis 28(14), 2358–2360 (2007)
B. Gimenez, A. Aleman, M.C. Guillen, Antioxidant activity of several marine skin gelatins. LWT- Food Sci. Technol. 44(2), 407–413 (2011)
J.Y. Je, C.B. Ahh, Y.J. Jeon, Y.T. Kim, Angiotensin I converting enzyme (ACE) inhibitory peptides from salmon byproduct protein hydrolysate by Alcalase hydrolysis. Process Biochem. 44(12), 2240–2245 (2012)
Y. Shai, From innate immunity to denovo designed antimicrobial peptides. Curr. Pharm. Des. 8(9), 715–725 (2002)
A. Connolly, O.P. Charles, R.J. FitzGerald, In vitro α-glucosidase, angiotensin converting enzyme and dipeptidyl peptidase-IV inhibitory properties of brewers’ spent grain protein hydrolysates. Food Res. Int. 56, 100–107 (2014)
K. Tsumara, T. Saito, K. Tsuge, H. Ashida, W. Kugimiya, K. Inouye, Functional properties of soy protein hydrolysates obtained by selective proteolysis. Lebensmittel-Wissenschaft & Technologie 38, 255–261 (2005)
K.N. Pearce, J.E. Kinsella, Emulsifying properties of proteins: Evaluation of a turbidimetric technique. J. Agric. Food Chem. 26, 716–723 (1978)
M.M. Hashim, D. Mingsheng, M.F. Iqbal, C. Xiaohong, Ginger rhizome as a potential source of milk coagulating cysteine protease. J. Phytochem. 72(6), 458–464 (2011)
A. Kumar, J. Sharma, A.K. Mohanty, S. Grover, V.K. Batish, Purification and characterization of milk Clotting enzyme from goat (Capra hircus). Comparat. Biochem. Physiol. Part B 145(1), 108–113 (2006)
H.R. Gheisari, H. Golkari, S.S. Shekarforoush, M. Aminlari, M. Raeisi, Possibility of Biarumcarduchcorum application as vegetable rennet in production of Iranian white cheese. J Food Hygiene 7, 27 (2017)
I.A. Jayawardana, M.J. Boland, K. Higgs, M. Zou, T. Loo, W.C. Mcnabb, C.A. Montoya, The kiwifruit enzyme actinidin enhances the hydrolysis of gluten proteins during simulated gastrointestinal digestion. Food Chem. 341(1), 128239 (2020)
C.F. Chi, Z.H. Cao, B. Wang, F.Y. Hu, Z.R. Li, B. Zhang, Antioxidant and functional properties of collagen hydrolysates from Spanish Mackerel skin as influenced by averagemolecular weight. Molecules 19, 11211–11230 (2014)
H. Zhuang, N. Tang, Y. Yuan, Purification and identification of antioxidant peptides from corn gluten meal. J. Funct. Foods 5(4), 1810–1821 (2013)
Y.Y. Ngoh, C.Y. Gan, Enzyme-assisted extraction and identification of antioxidative and α amylase inhibitory peptides from Pinto beans (Phaseolus vulgaris cv. Pinto). Food Chem. 1(190), 331–337 (2016)
A.C. Neves, P.A. Harnedy, M.B. O’Keeffe, FitzGerald RJ Bioactive peptides from Atlantic Salmon (Salmo salar) with angiotensin converting enzyme and dipeptidyl peptidase IV inhibitory, and antioxidant. Food Chem. 218(1), 396–405 (2017)
I.W. Cheung, S. Nakayama, M.N. Hsu, A.G. Samaranayaka, E.C. Li-Chan, Angiotensin-I converting enzyme inhibitory activity of hydrolysates from oat (Avena sativa) proteins by in silico and in vitro analyses. J. Agric. Food Chem. 57(19), 9234–9242 (2009)
J. Théolier, I. Fliss, J. Jean, R. Hammami, Antimicrobial peptides of dairy proteins: from fundamental to applications. Food Rev. Intl. 30(2), 134–154 (2014)
S.F. Taha, S.S. Mohamed, M.S. Wagdy, F.G. Mohamed, Antioxidant and antimicrobial activities of enzymatic hydrolysis products from sunflower protein isolate. World Appl. Sci. J. 21(5), 651–658 (2013)
X. Cheng, X. Tang, Q. Wang, X.Y. Mao, Antibacterial effect and hydrophobicity of yak κ-casein hydrolysate and its fractions. Int. Dairy J. 31(2), 111–116 (2013)
S.R. Pritchard, M. Phillips, K. Kailasapathy, Identification of bioactive peptides in commercial Cheddar cheese. Food Res. Int. 43(5), 1545–1548 (2010)
L. Zheng, H. Yu, H. Wei, Q. Xing, Y. Zou, Y. Zhou, Peng J Antioxidative peptides of hydrolysate prepared from fish skin gelatin using ginger protease activate antioxidant response element-mediated gene transcription in IPEC-J2 cells. J. Funct. Foods 51, 104–112 (2018)
M. Mahdabi, S.S.P. Hosseini, A comparative study on some functional and antioxidant properties of kilka meat, fishmeal, and stick-water protein hydrolysates. J. Aquat. Food Prod. Technol. 27, 844–858 (2018)
P. Viji, T.S. Phannendra, D. Jesmi, B.M. Rao, D.P.H. Das, N. George, Functional and antioxidant properties or gelatin hydrolysates prepared from skin and scale of Sole Fish. J. Aquat. Food Prod. Technol. 28, 976–986 (2019)
M. Hajfathalian, S. Ghelichi, M.P.J. García, S.A.D. Moltke, C. Jacobsen, Peptides: production, bioactivity, functionality, and applications critical review. Food Sci. Nutr. 58, 3097–3129 (2018)
A.N. Razali, A.M. Amin, N.M. Sarbon, Antioxidant activity and functional propierties of antioxidant activity and functional properties of fractionated cobia skin gelatin hydrolysate at different molecular weight. Int. Food Res. J. 22, 651–660 (2015)
L. Alolod, A. Garner, N.S. Nuñal, G. Nillos, G. Mae, P.J. Peralta, Bioactivity and functionality of gelatin hydrolysates from the skin of Oneknife Unicornfish (Naso thynnoides). J. Aquat. Food Prod. Technol. 28, 1013–1026 (2019)
N.R.A. Halim, H.M. Yusof, N.M. Sarbon, Functional and bioactive properties of fish protein hydolysates and peptides: A comprehensive review. Trends Food Sci. Technol. 51, 24–33 (2016)
C.M. Galanakis, T.M.S. Aldawoud, M. Rizou, N.J. Rowan, S.A. Ibrahim, Food ingredients and active compounds against the coronavirus disease (COVID-19) pandemic: a comprehensive review. Foods 9(11), 1701 (2020)
C.M. Galanakis, M. Rizou, T.M.S. Aldawoud, I. Ucak, N.J. Rowan, Innovations and technology disruptions in the food sector within the COVID-19 pandemic and post-lockdown era. Trends Food Sci. Technol. 110, 193–200 (2021)
M.C. Galanakis, The food systems in the era of the coronavirus (COVID-19) pandemic crisis. Foods 9(4), 523 (2020)
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
FK: Conceptualization, Investigation, Writing-Original Draft. MHA: Supervision, Funding acquisition. MR: Supervision, Resources, Writing-Review & Editing. RMN: Methodology, Editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest regarding the publication of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Khosravi, F., H, M., Azizi et al. Assessment of the biotechnological activity of wheat hydrolysates prepared with the Biarum bovei extract. Food Measure 16, 2738–2748 (2022). https://doi.org/10.1007/s11694-022-01379-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11694-022-01379-1