Abstract
Isobutylammonium hydrogen oxalate hemihydrate (IHOH), an organic nonlinear optical (NLO) crystal, is developed in this work using a solvent evaporation process. The single-crystal XRD (SCXRD) study reveals the monoclinic system and centrosymmetric space group C2/c of IHOH. The highly crystalline nature of IHOH is confirmed by the presence of well-defined peaks in the powder x-ray diffraction (PXRD) pattern. The presence of various functional groups in IHOH is ascertained by vibrational analysis. The lower cutoff wavelength of IHOH is 223 nm, and it is transparent up to 1100 nm. The refractive index (n), extinction coefficient (k), optical absorption coefficient (α) and optical bandgap (Eg) are determined. Nuclear magnetic resonance (NMR) spectral analysis establishes the molecular structure of IHOH. Z-scanning is employed to analyse the NLO characteristics of IHOH crystal. The crystal demonstrates saturable absorption and a self-defocusing nature. The third-order NLO susceptibility (χ3) of IHOH is calculated as 2.6718 × 10−7 esu. The presence of N–H···O and O–H···O bonds in the IHOH crystal is the reason for the high χ3 value. The photoluminescence (PL) spectrum reveals violet and blue emission from the crystal. The decomposition of IHOH is investigated using thermogravimetry/differential thermal analysis (TG/DTA analysis. The results reveal the suitability of IHOH for Q-switching, optical pulse shortening, optical sensors, and photonic and optoelectronic applications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nonlinear optical (NLO) crystals have garnered considerable interest because of their use in photonic devices.1,2 NLO materials are used in optical modulation, frequency shifting, data storage, telecommunication and signal processing. Organic NLO crystals possess high NLO efficiency and structural flexibility and are used in photonic devices. They exhibit excellent NLO properties, higher laser damage threshold and can be easily synthesized.3 Organic NLO crystals are used in optical switching, logical devices and biological imaging.4,5 The ability to adjust the chemical structure with a diverse range of physical characteristics for the desired NLO properties is one of the main benefits of organic materials.6 Charge transfer can be easily achieved in these materials. All these characteristics make organic crystals suitable for photonic devices and are studied extensively.7,8,9,10,11,12
Carboxylic acids have received considerable attention in the domain of crystal growth technology due to their ability to form strong hydrogen bond interactions with the combining compound, resulting in various crystal structures.13 The solution growth technique is cost-effective and can produce single crystals of superior quality with fewer imperfections, as the growth occurs close to equilibrium conditions. Oxalic acid molecules possess the ability to self-assemble through extensive hydrogen bonding.14 Isobutylamine is an organic substance which falls under the category of amines. It consists of an amino group (–NH2) linked to a carbon atom that is directly bound to three hydrogen atoms, one alkyl group known as isobutyl and three hydrogen atoms, making it a primary amine. Four carbon atoms are arranged in an expanding chain to form the isobutyl group. In the structure of isobutylammonium oxalate hemihydrate, oxalate ions are joined by O–H···O bonds and are linked to isobutylammonium ions and water molecules by N–H···O bonds.15
There have been various reports of oxalic acid-based crystals. A series of NLO active materials composed of oxalic acid have been synthesized, analysed and reported, including diisopropylammonium oxalate, allylammonium hydrogen oxalate hemihydrate, ethylammonium hydrogen oxalate hemihydrate, benzylammonium hydrogen oxalate hemihydrate, diethylammonium hydrogen oxalate, 2,2′-bipyridine-1-ium hemioxalate oxalic acid monohydrate, ethyldimethylammonium oxalate, bis(tert-butyl-ammonium) oxalate, dimethylammonium oxalate, 1,2,4-triazolium hydrogen oxalate, 1,3-benzothiazole-oxalic acid, cytosinium oxalate monohydrate, 2-methyl-1H-imidazol-3-ium hydrogen oxalate dihydrate, dipyridinium oxalate-oxalic acid, pyridinium and morpholinium hydrogen oxalate salts, creatininium oxalate monohydrate and (ferrocenylmethyl)dimethylammonium hydrogen oxalate.16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32
The growth and characterization of certain oxalic acid-based crystals has been reported by our group.13,33,34 In this work, isobutylammonium hydrogen oxalate hemihydrate (IHOH) is grown by slowly evaporating the solvent at room temperature, and the product is studied by single crystal x-ray diffraction (SCXRD), powder x-ray diffraction (PXRD), ultraviolet–visible–near infrared (UV-Vis-NIR) spectroscopy, Fourier transform infrared (FT-IR) and FT-Raman spectroscopy, nuclear magnetic resonance (NMR) spectroscopy, photoluminescence (PL), thermogravimetry/differential thermal analysis (TG/DTA) and Z-scan analysis to determine the suitability of IHOH for photonic applications.
Materials and Methods
The title compound was grown by reacting an equimolar ratio of isobutylamine (Thermo Scientific; 99%) and oxalic acid dihydrate (Loba; 99.5%) in distilled water, and the solution was stirred well using a magnetic stirrer for about 2 h to obtain a homogeneous saturated solution. This saturated solution was filtered using Whatman filter paper. The filtered solution was then covered using polythene paper, and a few holes were made for solvent evaporation, after which the solution was stored in a dust-free environment. Slow evaporation leads to supersaturation and growth of crystals. Good-quality crystals were harvested after 2 weeks and are shown in Fig. 1. The equation for crystal formation can be written as follows.

Results and Discussion
SCXRD Analysis
Structural analysis of IHOH was performed using a Bruker Enraf-Nonius CAD4 diffractometer (λ = 0.7106 Å). The crystal system was found to be monoclinic and belonging to space group C2/c. The space group is centrosymmetric and has the potential to exhibit third-harmonic generation. The lattice constants of IHOH crystal are a = 21.866 (7) Å, b = 5.6406 (19) Å, c = 16.605 (5) Å and β = 119.10 (2)°, and the unit cell volume is 1793 Å3. The observed data agree well with the values in the literature.15
PXRD Analysis
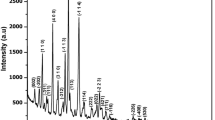
PXRD study was performed using a PANalytical X'Pert PRO X-ray diffractometer (λ = 1.54060 Å). Diffraction peaks were indexed by IndX software. Miller indices and interplanar distances were used to determine the lattice constants using UnitCell software. The diffraction pattern shown in Fig. 2 reveals the highly crystalline nature of IHOH. The calculated cell constants match well with reported values.15 Cell parameters deduced from SCXRD and PXRD analysis and the reported values are given in Table I.
FT-IR and FT-Raman Analysis
The FT-IR and FT-Raman spectra of IHOH are given in Figs. 3 and 4, respectively. Vibrational assignments are presented in Table II.
The broad absorption peaks present between 3600 cm−1 and 2800 cm−1 in IR and 3200 cm−1 and 2800 cm−1 in Raman spectroscopy are assigned to OH, CH2, CH3 and NH3+ stretching vibrations of IHOH. The OH bending vibration produces peaks near 1719 cm−1 and 1702 cm−1 in IR and 1713 cm−1 in Raman spectroscopy. The OH out-of-plane bending vibration is observed at 720 cm−1in IR and 707 cm−1 in Raman spectroscopy. The intense signal observed at 1631 cm−1 in IR and 1602 cm−1 in Raman spectroscopy is attributed to C=O stretching. The presence of COO− stretching vibration is confirmed near 1503 cm−1, 1471 cm−1 and 1272 cm−1 in IR and 1469 cm−1 in Raman spectroscopy. The absorption peak occurring at 1338 cm−1 in IR and 1358 cm−1 in Raman spectroscopy is due to stretching of C–N. The CH2 stretching vibration is observed at 1318 cm−1 in IR and 1319 cm−1 in Raman spectroscopy. C–O stretching is confirmed by the absorption peak near 1230 cm−1 in IR and 1200 cm−1 in Raman spectroscopy. The in-plane bending vibration of CH2 is around 1161 cm−1 in IR and 1171 cm−1 in Raman spectroscopy. CH3 rocking is observed at 1109 cm−1 in IR and 1098 cm−1 in Raman spectroscopy. C–C stretching occurs at 953 cm−1, 878 cm−1 and 858 cm−1 in IR and 966 cm−1, 935 cm−1, 822 cm−1 and 804 cm−1 in Raman spectroscopy. The peak occurring at 786 cm−1 in IR and 784 cm−1 in Raman spectroscopy is due to rocking of CH. COO- wagging vibration is observed at 574 cm−1 in IR and 563 cm−1 in Raman spectroscopy. The C–N deformation mode appears around 498 cm−1 in IR and 492 cm−1 in Raman spectroscopy. The bending of C=O occurs near 460 cm−1 in IR and 486 cm−1 in Raman spectroscopy. Lattice modes are found at 197 cm−1, 130 cm−1 and 74 cm−1 in Raman spectroscopy. The NH2 group of isobutylamine is protonated (NH2 to NH3+) by the carboxylic group (COOH to COO−) of oxalic acid dihydrate. The crystal structure of IHOH crystal is built by a positively charged C4H12N+ group as donor and negatively charged C2HO4− group as acceptor. The presence of NH3+, COO− and OH functional group vibrations confirms the formation of the crystal. The assignment of vibrational bands is carried out using values in the literature.35,36,37,38,39,40,41,42,43,44,45,46,47,48
UV-Vis-NIR Spectral Analysis
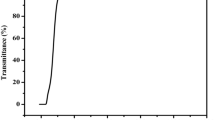
The UV-Vis-NIR spectrum of IHOH was obtained using a PerkinElmer spectrometer in the wavelength range of 190–1100 nm, and the observed optical transmission spectrum is given in Fig. 5. The lower cutoff wavelength lies at 223 nm. The spectrum reveals that IHOH crystal is transparent from 223 nm to 1100 nm. IHOH shows excellent transmission in the visible portion, making IHOH suitable for optoelectronic and NLO devices. As the crystal is transparent from 223 nm, it can be used to create new wavelengths up to 223 nm by frequency conversion. Transmission in the visible region can be exploited for optoelectronic applications. As the spectrum covers the UV and IR regions, it can be used in optical devices and sensors.49
The value of α was determined using the formula
where T is the transmittance and t is the thickness of IHOH.
The value of Eg was determined by Tauc’s plot using the relation
where n is ½ for the allowed direct transition. A graph was drawn between hυ and (αhυ)2. Eg was determined by extrapolating the linear portion of the curve and was found to be 5.41 eV (Fig. 6). This confirms the direct-bandgap nature of IHOH crystal. This wide energy bandgap of IHOH confirms the transmission in the visible portion.
The reflectance (R) of IHOH was found using the formula,
k is the amount of electromagnetic energy lost due to scattering and absorption per unit thickness in a crystal and was found using,
Figures 7 and 8 display the spectra of k and n versus λ. It is clear that the values of n and k are strongly dependent on λ, mostly in the UV region. k values are high in the UV region, decreasing with an increase in λ, and remain constant in the visible region. This is a suitable characteristic for optical devices.50
The refractive index (n) of IHOH was calculated as follows:
The n of IHOH was found to be 1.4035. The n value in the UV-visible range confirms its suitability for optical components such as signal filters, reflectors and refractive coating in solar thermal devices.51
NMR Analysis
13C and 1H NMR spectra of IHOH crystal were obtained using a Bruker Avance spectrometer (400 MHz) and are shown in Figs. 9 and 10, respectively. The chemical shifts and group identifications are shown in Table III.
13C NMR
In pure isobutylamine, the CH2 and CH chemical shifts are reported at 50.22 ppm and 31.57 ppm, respectively.52 The carbon signal for the CH2 and CH groups present in the IHOH crystal are shifted upfield and found at 46.20 ppm and 26.85 ppm, respectively. The CH3 carbon signal is reported at 20.02 ppm for isobutylamine, and the same signal is observed at 20.15 ppm in IHOH crystal. The signal for carbon in the carboxylic group is reported at 162.31 ppm for pure oxalic acid dihydrate, and this peak is shifted and observed at 163.97 ppm in IHOH. The solvent peak appears at 39.82 ppm. The existence of N–H···O and O–H···O interactions in IHOH are the reason for the peaks observed at new positions.
1H NMR
The CH2 and CH group signals of pure isobutylamine are reported at 2.859 ppm and 1.959 ppm, respectively. These are shifted and found at 2.647 ppm and 1.906 ppm in IHOH, respectively. The CH3 chemical shift is observed at 0.926 ppm for IHOH, and the same is reported for isobutylamine at 0.998 ppm. Due to protonation, the NH2 peak that was noted at 1.48 ppm for pure isobutylamine is observed at 8.023 ppm.53 The water signal of IHOH is found at 6.924 ppm, and the water signal for oxalic acid dihydrate is reported at 8.2 ppm.54,55 The presence of N–H···O and O–H···O bonds shifts the signals to new positions and establishes the formation of IHOH crystal. The DMSO solvent signal is observed at 2.522 ppm.
Z-Scan Analysis
The NLO parameters of IHOH are evaluated by Z-scan analysis. This analysis is carried out utilizing a He-Ne laser (λ = 632.8 nm), and the incident intensity at the focus is 3.13 mW/cm−2, focused by a lens with focal length of 12 cm; the aperture of the beam radius ωa is 5 mm and the radius of the aperture (ra) is 4 mm.
The open- and closed-aperture plots are shown in Figs. 11 and 12, respectively. In the open-aperture graph, there is maximum transmission at the focus. This is due to saturable absorption, and makes the IHOH crystal suitable for laser Q-switching and optical pulse compression applications. In the closed-aperture graph, the pre-focal peak and post-focal valley reveal the negative nonlinear refractive index (−n2) of IHOH, which is due to its self-defocusing nature and makes it suitable for optical sensors such as night vision devices.56 Table IV shows the NLO constants of IHOH crystal, where the χ3 value of IHOH is 2.6718 × 10−7 esu. Table V compares the χ3 value of IHOH with that of the reported crystals AMBP4N, HQ2C1NB, HEANP, and DIPA-N.57,58,59,60 It can be seen that the χ3 value of IHOH is higher than the values of the other NLO crystals. The structure of IHOH consists of N–H···O, O–H···O bonds and is attributed to the large χ3 value. These bonds modify electronic charge distribution and increase the molecular polarization.
Photoluminescence Spectral Analysis
Photoluminescence emission is very sensitive to the defects present in crystals. In PL, the excited photons are reflected at different wavelengths and reveal details regarding the structural perfection of crystals. IHOH was excited at 223 nm, and the emission spectrum recorded using a Cary Eclipse fluorescence spectrometer is shown in Fig. 13. The spectrum shows a peak at 418 nm and two high-intensity peaks at 448 nm and 484 nm. The corresponding excitation energies are 2.96 eV, 2.76 eV and 2.56 eV, which reveal that IHOH has violet and blue emission. These emission colours are attributed to shallow defects present in the bandgap and to a more ordered structure.61
Thermal Analysis
TG/DTA analysis was performed to examine the thermal stability of IHOH. An alumina crucible was used to hold 1.43 mg of the crystalline sample, and a heating rate of 10 °C/min was employed to heat IHOH between 30 °C and 500 °C. Figure 14 depicts the TG/DTA curve of IHOH crystal. The TG curve demonstrates two stages of weight reduction. In the first stage, an initial weight loss of 9.33% occurs within the temperature range of 30–152 °C because of the release of water in the crystal lattice. The second stage takes place in the temperature range of 152–215 °C, which exhibits a weight loss of 89.01%. The weight loss is complete at 215 °C. This loss of weight is due to the complete decomposition of the crystal. The endothermic peaks visible at 152 °C and 180 °C in the DTA curve coincide with the weight loss. The final endothermic peak, which is seen at 202 °C, shows that thermal energy was utilized to fully destroy the sample in the holder.
Conclusion
IHOH crystals were developed by a slow solvent evaporation process at room temperature. The SCXRD analysis revealed that IHOH possesses a monoclinic system with a centrosymmetric C2/c space group. The lattice constants of IHOH crystal are a = 21.866 (7) Å, b = 5.6406 (19) Å, c = 16.605 (5) Å and β = 119.10 (2)°, and the unit cell volume is 1793 Å3. Well-defined peaks in the PXRD pattern establish the highly crystalline nature of the title crystal. IHOH possesses good optical transparency between 223 nm and 1100 nm and shows excellent transmission in the UV-Vis-NIR range, making it suitable for optoelectronic and NLO devices. The bandgap of IHOH was found to be 5.41 eV. The presence of functional groups and their corresponding interactions was confirmed by FT-IR and FT-Raman spectral analysis. NMR spectroscopy was used to establish the molecular structure. The shift in the peak positions of water, NH2 and other groups when compared to the parent molecules established the formation of the title crystal. The third-order NLO behaviour of IHOH was ascertained from Z-scan analysis, which revealed that the crystal is self-defocusing and exhibits saturable absorption. Z-scan analysis was employed to determine χ3 as 2.6718 × 10−7 esu. This is attributed to the presence of N–H···O and O–H···O hydrogen bonds in IHOH. The observed results reveal the suitability of IHOH for laser Q-switching, optical pulse shortening and optical sensors such as night vision devices. The photoluminescence spectrum shows violet and blue emission. The TG/DTA results show that IHOH is stable only at room temperature. The obtained results reveal that IHOH is suitable for use in photonic and optoelectronic applications.
Data Availability
Data will be made available on request.
References
M. Jazbinsek, L. Mutter, and P. Gunter, Photonic applications with the organic nonlinear optical crystal DAST. IEEE J. Quantum Electron. 14, 1298 (2008).
P.N. Prasad, and D.J. Williams, Introduction to nonlinear optics effect in molecules and polymers (New York: John Wiley, 1991).
K. Ambujam, S. Selvakumar, P. Ginson Joseph, I. Vetha Potheher, and P. Sagayaraj, thermal, optical, electrical properties of gel grown ZMTC. Mater. Manuf. Proc. 22, 351 (2007).
H.L. Bhat, Growth and characterization of some novel crystals for nonlinear optical applications. Bull. Mater. Sci. 17, 1233 (1994).
G. Gokila, R. Aarthi, and C. Ramachandra Raja, Structural, spectral and nonlinear optical analysis of Bis (2-methyllactato) borate tetrahydrate: a new nonlinear optical crystal for laser applications. Optik 207, 1 (2020).
M. Shkir, B. Riscob, and G. Bhagavannarayana, Synthesis, growth, structural, spectroscopic, crystalline perfection, second harmonic generation (SHG) and thermal studies of 2-aminopyridinium picrate (2APP): a new nonlinear optical material. Solid State Sci. 14(7), 773–776 (2012).
D.S. Chemla, and J. Zyss eds., Nonlinear optical properties of organic molecules and crystals. (New York: Academic Press, 1987).
J. Badan, R. Hierle, A. Perigaud and J. Zyss, Nonlinear optical properties of organic molecules and polymeric materials. American chemical symposium series 233, American chemical society (1993).
R.A. Hann and D. Bloor, Organic materials for nonlinear optics (Cambridge: The Royal Society of Chemistry, 1989).
V.G. Dmitriev, G.G. Gurzadyan, and D.N. Nicogosyan, Handbook of nonlinear optical crystals (New York: Springer-Verlag, 1999).
J. Zyss, J. Pecaut, J.P. Levy, and R. Masse, sysntheisi and crystal structure of guanidinium L- monohydrogen tartrate: encapsulation of an optically nonlinear octupolar cation. Acta Cryst. 49, 334 (1993).
P.S. Patil, P.A. Kumar, S.V. Rao, and G. Bhagavannarayana, growth and characterization of a new organic nonlinear optical crystal: 1-(3-Nitrophenyl)-5-phenylpenta-2, 4-dien-1-one. Opt. Laser Technol. 71, 108–113 (2015).
P. Deepa, R. Aarthi, S. Kalainathan, and C. Ramachandra Raja, Synthesis, structural, spectral and third-order nonlinear optical analysis of solution-grown 4-methylbenzylammonium oxalate hydrate: a novel organic crystal for laser applications. J. Mater. Sci. Mater. Electron. 34, 925 (2023).
S. Jeganathan, S. Ilayabarathi, S. Tamilselvan, and R. Mohan, Growth and characterization of nonlinear optical crystal: oxalic acid dihydrate. J. Cryst. Growth 312, 3006 (2010).
B. Dziuk, B. Zarychta, and K. Ejsmont, Crystal structure of isobutylammonium hydrogen oxalate hemihydrate. Acta Cryst. E70, o1175 (2014).
M. Vij, H. Yadav, S. Goel, N. Vashistha, D. Nayak, P. Kumar, and K.K. Maurya, Crystal structure, High-resolution X-ray diffraction and Hirshfeld surface analysis of a novel third-order nonlinear optical crystal: Diisopropylammonium oxalate. J. Mol. Struct. 1246, 131177 (2021).
B. Dziuk, B. Zarychta, and K. Ejsmont, Allylammonium hydrogen oxalate hemihydrate. Acta Cryst. 70(8), o852–o852 (2014).
K. Ejsmont, and J. Zaleski, Ethylammonium hydrogen oxalate hemihydrate. Acta Cryst. E62, o3879 (2006).
J.C. Barnes, Benzylammonium hydrogen oxalate hemihydrate. Acta Cryst. E59, o931 (2003).
K. Ejsmont, Diethylammonium hydrogen oxalate. Acta Cryst. E63, o107 (2007).
B. Dziuk, and A. Jezuita, 2,2׳-Bipyridin-1-ium hemioxalate oxalic acid monohydrate. IUCr DATA 3, x181219 (2018).
K. Ejsmont, Ethyldimethylammonium oxalate. Acta Cryst. E62, o5852 (2006).
K. Ejsmont, and J. Zaleski, Bis(tert-butylammonium) oxalate. Acta Cryst. E62, o2512 (2006).
W. Diallo, NadongoGueye, Aurelien Crochet, Lauerent Plasseraud and Helene Cattey, Crystal structure of dimethylammonium hydrogen oxalate hemi(oxalic acid). Acta Cryst. E71, 473 (2015).
N. Ponjan, P. Aroonchat, and K. Chainok, Crystal structure and Hirshfeld surface analysis of 1, 2, 4-triazolium hydrogen oxalate. Acta Cryst. 76(2), 137–140 (2020).
A.A.A. Abdalsalam, M.T.M. Al-Dajani, N. Mohamed, M. Hemamalini, and H.K. Fun, 1,3-Benzothiazole-oxalic acid (2/1). Acta Cryst. E 67, o2342 (2011).
K. Bouchouit, N. Benali-Cherif, S. Dahaouil, E.E. Bendeif, and C. Lecomte, Cytosinium oxalate monohydrate. Acta Cryst. E61, o2755 (2005).
M.B. Diop, L. Diop, L. Plasseraud, and H. Cattey, crystal structure of 2-methyl-1H-imidazol-3-ium hydrogen oxalate dihydrate. Acta Cryst. E72, 1113 (2016).
G.R. Newkome, K.J. Theriot, and F.R. Fronczek, dipyridinium oxalate- oxalic acid. Acta Cryst. C41, 1642 (1985).
Z.T. David, E.C. Mulrooney, R.F. Madden, V.D. Lonergan, H.M.-B. Slyusarchuk, and T.D. Keene, Hydrogen-bonding chain and dimer motifs in pyridinium and morpholinium hydrogen oxalate salts. Acta Cryst. E 74, 1804 (2018).
S. Asath Bahadur, R.S. Kannan, and B. Sridhar, Creatininium hydrogen oxalate monohydrate. Acta Cryst. E 63, o2387 (2007).
A. Mamadou Ndiaye, L.D. Samb, and T. Maris, Crystal structure of (ferrocenylmethyl)dimethylammonium hydrogen oxalate. Acta Cryst. E 71, 947 (2015).
K. Saranya, E. Muhildharani, and C. Ramachandra Raja, Elucidation of structural and third-order nonlinear optical characteristics of allylammonium hydrogen oxalate hemihydrate single crystal. J. Mater. Sci. Mater. Electron. 34, 688 (2023).
K. Saranya, E. Muhildharani, and C. Ramachandra Raja, Studies on the growth, structural, spectral and third order nonlinear optical properties of Ethylammonium hydrogen oxalate hemihydrate crystal. J. Mater. Sci. Mater. Electron. 34, 1211 (2023).
S. Sudha, P. Jayaprakash, C.R.T. Kumari, M. Nageshwari, G. Vinitha, R.G. Raman, and M.L. Caroline, Growth and characterization of Catena-((μ4-dihydrogen oxalate)-bis (μ3-hydrogen oxalato)-(μ2-dihydrogen oxalate)-tetra-aqua-di-rubidium){Rb (HC2O4)(H2C2O4)(H2O) 2} 1∞ single crystal for nonlinear optical applications. Chin. J. Phys. 67, 37–51 (2020).
P. Prabu, B. Vijayabhaskaran, A. Antony Joseph, R. Aarthi, and C. Ramachandra Raja, Structural, spectral and nonlinear optical analysis of aquachloro (l-prolinato)copper(II) crystal. J. Mater. Sci. Mater. Electron. 30, 5074 (2019).
G. Gokila, R. Aarthi, and C. Ramachandra Raja, Structural, spectral and optical characterization of Potassium bis(2-methyllactato)borate hemihydrate crystal. J. Electronic Mater. 49, 6130 (2020).
L. Wang, G. Zhang, X. Liu, X. Wang, L. Wang, and L. Zhu, Crystal growth, structure and spectroscopic studies of a novel organic single crystal: L-lysine p-nitrophenolate monohydrate. Cryst. Res. Technol. 48, 1087 (2013).
S. Natarajan, S.A. Martin Britto, and E. Ramachandran, Growth, Thermal, Spectroscopic and Optical studies of L-Alaninium Malate, a New Organic Nonlinear Optical Material. Cryst. Growth Design. 6, 137 (2006).
G. Gokila, R. Aarthi, and C. Ramachandra Raja, Structural, spectral and nonlinear optical analysis of Bis(2-methyllactato)borate tetrahydrate: a new nonlinear optical crystal for laser applications. Optik 207, 1 (2020).
K. Sivakumar, M. Senthilkumar, and C. Ramachandra Raja, Molecular, structural and optical properties of S-alanine S-mandelic acid crystals. Int. J. Res. 5, 2938 (2018).
N. Kalaimani, K. Ramya, R. Aarthi, and C. Ramachandra Raja, Growth, structural, optical, spectral and thermal characterization of third order nonlinear optical crystal: Diammonium fumarate. Chine. J. Phys. 61, 104 (2019).
K. Sivakumar, M. Senthilkumar, and C. Ramachandra Raja, A study on the structural, spectral and nonlinear optical properties of R-Phenylalanine-S-Mandelic acid single crystals. Int. J. Res. 5, 84 (2018).
R. Aarthi, P. Umarani, and C. Ramachandra Raja, Molecular structural confirmation and influence of hydrogen bond on third order nonlinear properties of bis(4-methylbenzylammonium) tetra chloride cadmate (II)single crystal. Optik 164, 449 (2018).
N. Kalaimani, R. Aarthi, and C. Ramachandra Raja, Structural, spectral, thermal and nonlinear optical analysis of potassium tartrate hemihydrate crystal. J. Mater. Sci. Mater. Electron. 30, 7974 (2019).
G. Gokila, R. Aarthi, and C.R. Raja, Elucidation of the properties of Lithium bis (2-methyllactato) borate monohydrate crystal for laser applications. J. Mater. Sci. Mater. Electron. 31(9), 6956–6962 (2020).
N. Kalaimani, K. Ramya, G. Vinitha, R. Aarthi, and C. Ramachandra Raja, Structural, spectral, thermal and nonlinear optical analysis of anhydrous citric acid crystal. Optik 192, 162960 (2019).
K.J. Arun, and S. Jayalakshmi, Growth and characterization of glycinium oxalate crystals for nonlinear optical applications. J. Optoelectron. Adv. Mater- Rapid Commun. 2, 701 (2008).
B. Deepa, K. Gayathiridevi, M.K. Chakravarthi, A. Shajahan, B.S. Sree, M.I. Anees, and M. Habeeb, Slow evaporation technique to grow 3-Amino benzene sulfonic acid single crystal for Non-Linear optical (NLO) transmission. Mater. Today: Proc. 62, 2119–2123 (2022).
A. Rajeswari, G. Vinitha, and P. Murugakoothan, Investigation on optical, thermal, mechanical, dielectric and ferroelectric properties of non linear optical single crystal guanidinium manganese sulphate. J. Mater. Sci. Mater. Electron. 29, 10 (2018).
P. Karuppasamy, M.S. Pandian, P. Ramasamy, and S. Verma, Crystal growth, structural, optical, thermal, mechanical, laser damage threshold and electrical properties of triphenylphosphine oxide 4-nitrophenol (TP4N) single crystals for nonlinear optical applications. Opt. Mater. 79, 152 (2018).
https://sdbs.dp.aist.go.jp/sdbs/cgi-bin/directframe-top.cgi.
R. Aarthi, and C. Ramachandra Raja, Spectral and optical characterization of the new semi-organic crystal: 4-methylbenzylammonium chloride hemihydrate, to establish protonation and the effect of resultant hydrogen bonding. Bull. Mater. Sci. 42, 1 (2019).
Bo. Berglund, and R.W. Vaughan, Correlations between proton chemical shift tensors, deuterium quadrupole couplings, and bond distances for hydrogen bonds in solids. J. Chem. Phys. 73, 2037 (1980).
D. Vojta, M. Vrankic, M. Bertmer, and G.E. Schaumann, Dehydration of α- oxalic acid dehydrate: structural, spectroscopic and thermal study with implications on the disruption of water molecular bridges in soil organic matter. Thermochim. Acta 643, 73 (2016).
P.K. Sivakumar, S. Kumar, R.M. Kumar, R. Kanagadurai, and S. Sagadevan, Studies on growth, spectral, thermal, mechanical and optical properties of 4-bromoanilinium 4-methylbenzenesulfonate crystal: a third order nonlinear optical material. Mater. Res. 19, 937–941 (2016).
P. Karuppasamy, T. Kamalesh, K. Anitha, S.A. Kalam, M.S. Pandian, P. Ramasamy, and S.V. Rao, Synthesis, crystal growth, structure and characterization of a novel third order nonlinear optical organic single crystal: 2-amino 4, 6-dimethyl pyrimidine 4-nitrophenol. Opt. Mater. 84, 475–489 (2018).
R. Mohan Kumar, and K. Ruckmani, Investigations on physicochemical and third-order nonlinear optical studies on quinolinium based organic crystal. J. Nonlinear Opt. Phys Mater. 28, 1 (2019).
N. Sudharsan, V. Krishanakumar, and R. Nagalakshmi, Synthesis, experimental and theoretical Studies of 8-hydroxyquinolinium 3,5-dinitrobenzoate single crystal. J. Cryst. Growth 398, 45 (2014).
M. Vij, Sonia, Harsh Yadav, Nikita Vashistha and Prashant Kumar, Crystal growth, structure and Z-scan studies on novel diisopropylammonium nicotinate crystal. J. Mol. Struct. 1206, 127759 (2020).
M. Vij, H. Yadav, N. Vashistha, M. Kumari, H.K. Verma, P. Kumar, and K.K. Maurya, Crystal structure, Hirshfeld surface analysis and thermal behavior of diisopropylammonium succinate, a novel third-order nonlinear optical crystal. J. Mater. Sci. 55, 16900–16913 (2020).
Acknowledgments
The authors thank the Sophisticated Analytical Instruments Facility (SAIF), Indian Institute of Technology (IITM), Chennai, for providing single-crystal XRD and FT-Raman spectra and acknowledge the Instrumentation Centre of St. Joseph’s College, Trichy, for recording FT-IR and UV-Vis-NIR spectra. The scientific/technical support extended by Gandhigram Rural Institute for (NMR and TG/DTA) and Alagappa University, Karaikudi, for providing PL and PXRD analysis are acknowledged. Also, the authors place on record special thanks to Dr. D. Sastikumar, National Institute of Technology, Trichy, for providing them with the third-order nonlinear testing facility for Z-scan measurement.
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
All authors contributed to the literature survey, material preparation, characterization and interpretation. The first draft of the manuscript was well discussed by all and written by KS. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
We wish to confirm that there are no conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome.
Ethical Approval
Not applicable
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Saranya, K., Muhildharani, E. & Raja, C.R. Studies on the Growth, Spectral and Optical Characteristics of Isobutylammonium Hydrogen Oxalate Hemihydrate Single Crystals. J. Electron. Mater. 53, 2467–2476 (2024). https://doi.org/10.1007/s11664-024-10991-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11664-024-10991-4