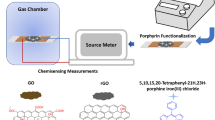
Abstract
The emergence of toxic pollutants due to heavy human intervention in the ecosystem causes serious environmental problems. Therefore, sensors based on material having a strong affinity towards specific environmental gaseous pollutants are urgently needed. The present study deals with chemiresistive gas sensors for the detection of sulfur dioxide (SO2) based on a composite of reduced graphene oxide (rGO) and 5,10,15,20-tetrakis(4-aminophenyl) porphyrin (TAPP). The improved Hummers method was used to synthesize graphene oxide (GO); it was further thermally reduced to rGO. The pattern of the copper electrode was coated on glass slides with a shadow mask using thermal evaporation. Then, GO was drop-cast between the two copper electrodes, thermally reduced to obtain rGO, and then modified by TAPP. The spectroscopic, structural, morphological, electrical, and optical studies were carried out using Fourier transform infrared spectroscopy, x-ray diffraction, Raman spectroscopy, atomic force microscopy, field emission scanning electron microscopy, current–voltage (I–V) and UV–visible spectroscopy, respectively. The developed sensor shows high selectivity towards SO2 gas analytes among exposed gaseous analytes. It exhibited reproducible response from 50 ppm to 200 ppm with enhanced repeatability at 50 ppm. The rGO/TAPP sensor exhibited a significant response (57 s) and recovery time (61 s), with a 5 ppm limit of detection.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The rapid growth of industrialization and urbanization has led to environmental contamination due to emerging pollutants, which represents a serious threat globally.1 Because of these issues, various hazardous toxic gases are becoming part of the environment, including sulfur dioxide (SO2),2 ammonia (NH3),3 nitrogen dioxide (NO2),4 carbon monoxide (CO),5 hydrogen sulfide (H2S),6 nitric oxide (NO),7,8 and volatile organic compounds (VOCs).9 Among these gases, SO2 is significantly increased in the environment due to the burning of fossil fuels and smelting of mineral ores. SO2 is a toxic, corrosive gas that contributes to various forms of environmental degradation, such as acid precipitation and haze formation.10 Overexposure to SO2 leads to several hazards to humans and ecosystems. SO2 can cause health issues such as bronchitis, and can irritate the nose, throat, and lungs. Moreover, it may exacerbate coughing, wheezing, phlegm, and asthmatic attacks. According to the Occupational Safety and Health Administration (OSHA), the US-recommended permissible exposure limit (PEL) is 5 ppm for monitoring of corrosive SO2 gas. Thus, humans need to be alert to the presence of corrosive gases in their surroundings. Many materials scientists have worked hard to fabricate innovative advanced materials that can detect corrosive gases with excellent selectivity, ideal stability, and sensitivity, and with a fast response and recovery time.
To date, many materials have been thoroughly studied for gas sensors, including conducting polymers,11 carbon-based materials,12 metal–organic frameworks (MOF),13,14 and metal oxides.15 Among the carbon family materials, reduced graphene oxide (rGO) is an oxygen-containing 2D carbon material that has attracted much attention in recent years due to its unique structure and excellent electronic, barrier, chemical, mechanical, optical, and thermal properties.16 Therefore, it has been widely explored in various sensor applications, supercapacitors, and solar cells. Its outstanding superior thermal conductivity, light transmittance, high Young's modulus, and high surface-to-volume ratio make it an excellent material for such applications.17 However, rGO has a significant issue in the field of sensors due to its high porosity, which leads to low recovery with non-selectivity.18,19,20
To overcome these issues, numerous research groups have continuously worked to overcome these hurdles. Recently, Li et al. reported rGO functionalized with hollow SnO2 nanofibers for an efficient sensor towards SO2, in which the device operates at room temperature (RT) with 5 ppm as the detection limit. However, response and recovery time are high, at 258 s and 150 s, respectively.21 A chemiresistive SO2 sensor based on GO-anchored poly(3,4-ethylenedioxythiophene)/poly(4styrenesulfonate), in which the sensor works at RT with a detection limit of 0.5 ppm, was reported by Sayyad et al.22 However, the reproducibility of the sensor has not been investigated. Shen et al. reported edge-tailored GO nanosheet-based field effect transistors for the detection of SO2 with response and recovery times of 7 s and 70 s, respectively.23 However, the limit of detection (LOD) was 275 ppm. rGO/WO3 and MWCNTs/WO3 nanocomposite-based sensors have been reported by Su et al. with response time and recovery time of 66 s and 298 s, respectively.24 The recovery time is on the higher side in this investigation. Zhai et al. reported a washable and flexible gas sensor based on a UiO-66-NH2 nanofiber membrane for the detection of SO2 with a response time of 255 s and recovery time of 170 s at 50 ppm. The sensor has demonstrated repeatability at 100 ppm, with linearity in the range of 125–1 ppm and stability for 30 days.25 However, the reproducibility aspect has not been investigated.
The development of materials to mitigate environmental concerns is a crucial step. Recently, porphyrin has been widely explored for such types of problems. Porphyrin is an aromatic macrocyclic organic functional group material widely studied for sensing applications. The schematics of porphyrin and TAPP porphyrin are indicated in Fig. 1. It has four pyrrole rings attached to four methane groups known as free-base porphyrin. In this free-base porphyrin ring, four meso-positions are 5, 10, 15, and 20, respectively; these meso-positions are attached to 4-aminophenyl functional group, known as meso-tetrakis (4-aminophenyl) porphyrin. The recent usage of porphyrins includes various fields such as cancer therapy, biomedical applications, supramolecular application, and high-capacity information-storage devices.26 Moreover, porphyrins capture widespread attention in the field of sensors,27 as porphyrin is an extremely attractive materials due to its unique structure, chemical stability, primarily influential selectivity, and sensitivity towards corrosive analytes.28,29 Zhu et al. reported a NO2 sensor based on 5-(4-ferrocamidophenyl)-10,15,20-triphenylporphyrin (H2FcPor) with a LOD of 1 ppm at RT with excellent sensitivity, good stability, and selectivity and response time of 120 s and recovery time of 600 s.30 However, the recovery time is on the higher side in this investigation. Shirsat et al. reported a FeTPP/rGO chemiresistive sensor for the detection of CO with a LOD of 2.5 ppm with a response time of 60 s and a recovery time of 120 s. The FeTPP/rGO sensor was found to be sensitive, with good repeatability, reproducibility, and excellent LOD towards CO gas.31 However, the stability of the sensor has not been investigated. Shaik et al. reported a chemiresistive gas sensor for sensitive detection of NO2 based on nitrogen-doped graphene nanosheets at RT with a LOD of 120 ppb and high selectivity at 100 ppm.32 However, the reproducibility aspect has not been investigated. As a derivative of porphyrin, TAPP is a versatile material that has functional diversity in various fields.33,34,35,36 Su et al. reported carbon nanotubes functionalized with porphyrin (FeTPP, FeOEP, NiTPP, and RuTPP) and phthalocyanine derivatives for detection of SO2 and NO2 with a LOD of 2 ppm.37 However, 5,10,15,20-tetrakis(4-aminophenyl)porphyrin (TAPP) has not been explored much. Moreover, there are very few reports on the investigation of materials for the selective detection of hazardous gas analytes. The present investigation deals with a chemiresistive-based gas sensor fabricated using rGO/TAPP for highly selective detection towards SO2 analyte. An improved version of the Hummers method was used to synthesize GO. GO to rGO conversion was successfully carried out using a thermal method. Synthesized materials were characterized for structural, morphological, spectroscopic, and electrical properties. The study indicates that the rGO/TAPP-based sensor exhibits fast response time and recovery time, with a LOD of 5 ppm, which is on par with the PEL of SO2 suggested by OSHA. The sensor exhibited reproducibility, repeatability, linearity, selectivity, sensitivity, and stability towards SO2. This material is well suited for developing sensing gadgets for real-time detection of environmental gaseous pollutants.
Experimental Part
Materials and Chemicals
Graphite powder was purchased from Loba Chemie Pvt Ltd (98% extra pure). Orthophosphoric acid (H3PO4) and potassium permanganate (KMnO4) were purchased from Thermo Fisher Scientific India Pvt Ltd. N,N-dimethylformamide (DMF), hydrogen peroxide (H2O2), and sulfuric acid (H2SO4) were obtained from Molychem, India. 5,10,15,20-Teterakis (4-aminophyenl)-porphyrin (TAPP) was purchased from TCI Co. Ltd.
Synthesis of GO
An improved Hummers method was utilized to synthesize GO powder.38,39 In brief, firstly, sulfuric acid was mixed with orthophosphoric acid (360 mL:40 mL). Then, as a second step, 3 g of graphite powder was added to the abovementioned solution. Next, potassium permanganate (KMnO4, 18 g) was slowly added to the solution. This solution was stirred continuously for up to 30 min at RT, followed by stirring for 48 h at 45°C. Subsequently, 1000 mL of deionized (DI) water in the form of ice was added to the solution after stirring. Then, after 5 min, 10 mL of hydrogen peroxide (H2O2) was slowly added. When the precipitate settled, the remaining solution was discarded, followed by residue solution washing three times using DI water and HCl (3:1 ratio). Finally, 1000 mL of DI water was used to wash the solution, and this step was repeated several times to attain normal pH. The final filtrates were dried and kept for further use.
Synthesis of rGO and Preparation of Substrate
Acetone and isopropyl alcohol were used to clean the glass slides, and then copper (with a thickness of 200 nm) was coated via thermal evaporation with a shadow mask to obtain patterned copper electrodes. Then, GO was drop-cast between the two electrodes and heated at 200°C on a hotplate for 5 min, resulting in the thermal reduction of GO to obtain rGO.40 Later, TAPP was drop-cast onto two similar rGO chips and dried at RT (22°C).
Functionalization of rGO/TAPP
TAPP (6.7 mg) was added to 10 mL DMF and subjected to ultrasonication for 10 min to form a uniform solution. Similarly, rGO/DMF was also subjected to ultrasonication. In brief, the gap between the two electrodes was bridged by aligning a 1-µl droplet of rGO and allowing it to dry at RT for 24 h. After drying, a 1-µl droplet of TAPP was drop-cast on the surface of rGO, and the substrate was kept for drying.
Surface and Electrical Characterization of the Sensors
X-Ray Diffraction (XRD)
The crystal structure of GO, rGO, TAPP, and rGO/TAPP was characterized using a Bruker D8 Advance x-ray diffractometer (Germany) with 40 mA current and 40 kV accelerating potential at a scanning rate of 5° min−1 in the range of 5° to 50° with CuKα1 radiation (λ = 1.54 Å).
Fourier Transform Infrared Spectroscopy (FTIR)
A Bruker Alpha FTIR in ATR mode (ZnSe window in the range of 900–4500 cm−1) was used to perform functional group studies of GO, rGO, TAPP, and rGO/TAPP.
UV–Vis Spectroscopy
UV–Vis spectra of GO, rGO, TAPP, and rGO/TAPP samples were recorded within the spectral range of 200–900 nm using a JASCO UV–Vis spectrophotometer.
Raman spectroscopy
Raman spectra of GO, rGO, TAPP, and rGO/TAPP were captured using an XploRA PLUS confocal raman microscope, which has a spectral range of 800–2000 cm−1.
Atomic Force Microscopy (AFM)
The AFM images and histogram of height distribution of GO, rGO, TAPP, and rGO/TAPP in non-contact mode with a 3.0 µm × 3.0 µm scan area were recorded using a Park XE-7.
Field Emission Scanning Electron Microscopy (FESEM)
The FESEM images of GO, rGO, TAPP and the rGO/TAPP composite were studied using a TESCAN MIRA3 LMH.
Electrical Characterization of the Sensors
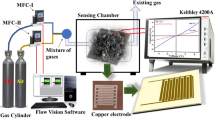
Electrical characterization data of rGO and rGO/TAPP were recorded using a Keithley 4200A semiconductor parameter analyzer. An in-house dynamic gas sensing system was employed for conducting gas sensing experiments. Schematic and physical diagram of the gas sensor characterization system is shown in Fig. 2. The real-time chemiresistive response was evaluated using a Keithley 4200A semiconductor parameter analyzer. Achieving precise manipulation of SO2 and dry air streams was possible using a programmable mass flow controller (MFC; Alicat Scientific, USA). This MFC demonstrated remarkable versatility in handling both non-corrosive and corrosive gases. Throughout the experiments, unless explicitly mentioned, ambient conditions were scrupulously upheld at RT, accompanied by a consistently maintained relative humidity of 30%. This humidity level was closely monitored using a dedicated humidity sensor in tandem with a digital temperature sensor.
Equation 1 serves as a concise representation of the sensor responsive behavior.
Rgas denotes the resistance of the sensor when exposed to the target gas (SO2 in this case) and Rair signifies the resistance of the sensor in an air environment. The metrics of response time and recovery time were defined; the former as the interval necessary to attain 90% of the saturated response, and the latter as the time essential for restoring the device to its baseline resistance.
Results and Discussion
X-Ray Diffraction (XRD)
XRD patterns for GO, rGO, TAPP, and rGO/TAPP are shown in Fig. 3a. The diffraction peak for GO at 2θ is 10.83°, and the d spacing is 0.81 nm due to intercalation of the oxide functional group at the carbon basal plane, such as oxygen-containing functional groups between layers. Additionally, the diffraction peak appeared at around 42.50°. The XRD pattern of GO indicates that GO exhibits turbostratic disorder,1 demonstrating that the improved Hummers method successfully synthesized GO. After the thermal reduction of GO, the XRD spectra show a peak at 2θ is 24.32° (d spacing 0.36 nm) and another peak at 12.06° (d spacing 0.73 nm) in the rGO XRD pattern is observed, which indicates the absence of oxygen-containing functional groups. Moreover, this small peak indicates the successful synthesis of partial rGO via thermal reduction.41 The XRD spectra for TAPP revealed peak positions 3.50°, 29.28°, 39.25°, 43.16°, 47.41°, and 48.55°. Comparing rGO/TAPP spectra with rGO and TAPP, both entities are found in the composite but 12.06° peak position in rGO, is disappeared in the spectra of rGO/TAPP composite and also 24.32° peak of rGO also shows a red shift in the spectra of rGO/TAPP. This indicates that the composite of rGO/TAPP was successfully formed via the facile drop-casting technique.
Fourier Transform Infrared Spectroscopy (FTIR)
The FTIR spectroscopy (in attenuated total reflectance (ATR) mode) is used to confirm the functional groups of all prepared samples GO, rGO, TAPP, and rGO/TAPP within the range of 500–4000 cm−1 using a Bruker FTIR spectrometer, shown in Fig. 3b. The number of oxygen-containing functional groups in GO, including oxygen, carboxylic, hydroxyl, epoxide, and carbonyl groups, are introduced as a peak point in the GO spectrum. The peak at 2349 cm−1 is related to atmospheric CO2, and the presence of absorbed water molecules and alcohol groups causes the absorption peaks that correspond to OH stretching (a peak 3334 to 3203 cm−1), which is superimposed over the OH stretch of carboxylic acid.3 The 1719 cm−1 peak indicate the incidence of oxidation C=O and C–O stretching vibration in COH. The band at 1621 cm−1 exhibits the C=C sp2 hybridized stretching vibration along with the N–H in-phase bending. The 1368 cm−1, 1229 cm−1, and 1040 cm−1 peaks result from the connection between C–N stretching and N–H bending, C–O stretching vibration of C–O in COC, and the C–O–C epoxide group.42 This means the synthesis of GO has been done successfully. Comparing GO, rGO, TAPP, and rGO/TAPP spectra, in the rGO spectrum, we can see the absence of an oxygen-containing functional group compared to the GO spectrum, indicating successful synthesis of rGO. As it is seen in the TAPP spectrum, the peak located at 3609 cm−1, 3550 cm−1, and 1031 cm−1 are related to OH, NH, and NO2, respectively.43 As a comparison between rGO and rGO/TAPP spectra, the absence of a NO2 band in the rGO/TAPP spectrum reveals the successful coating of TAPP on the surface of rGO. The additional peak at 3622 cm−1 is related to the NH band and high intensity compared to rGO. This might be because of the stretching vibration overlap of the NH, OH, and NO2 of TAPP and rGO, respectively.
UV–Vis Spectroscopy
UV–Vis spectra of GO, rGO, TAPP, and rGO/TAPP samples were recorded within the spectral range of 200 nm to 900 nm using a JASCO UV–Vis spectrophotometer. As shown in Fig. 3c, the GO spectrum shows two significant absorbance peaks at 230 nm and a shoulder peak at 303 nm. These two peaks represent the π–π* transition of aromatic rings in the C=C bond and n–π* transition of C=O from the oxidized carbon of GO.44 The π–π* transition peak in the rGO (rGO in dashed line) spectrum displays a red shift from 230 nm to 270 nm due to the removal of the oxygen-containing functional group with the absence of n–π* transition peak, which indicates that rGO was successfully synthesized via the thermal method.45 In the dash-dotted line, the TAPP spectrum shows the absorption peak positions or band positions in the spectrum of TAPP at 434 nm, 526 nm, 574 nm, and 665 nm, which are very close to the reported data.46 Comparing these four peaks, 434 nm has high absorption because of the transition of the electronic state, representing S0 to S2, known as the Soret band or B band.47 The rGO/TAPP composite spectral peaks position at nearly 430 nm and low absorption peaks at 526 nm, 574 nm, and 665 nm are due to the transition of the electronic state of S0 to S1 ranging from 500 nm to 700 nm, known as the Q band.48,49 Comparing the three rGO, TAPP, and rGO/TAPP composite spectra, we can see that the rGO/TAPP composite has been synthesized successfully.50
The band gap energy of GO, rGO, TAPP, and rGO/TAPP was calculated from the UV–Vis spectra of respective materials. The optical band gap of GO, rGO, TAPP, and rGO/TAPP is shown in Fig. 4. The optical bang gap observed for rGO is 1.5 eV, which resembles that reported earlier.5 A higher optical bang gap is observed for GO which is 3.65 eV. At the same time, the optical band gap for the rGO/TAPP composite is 2.4 eV, less than TAPP (2.6 eV). This will be helpful in sensing applications.
Raman Spectroscopy
Raman spectra of GO, rGO, TAPP, and rGO/TAPP were captured using an XploRA PLUS confocal Raman microscope, which has a spectral range of 800–2000 cm−1, as shown in Fig. 3d. The Raman spectrum for GO has two prominent significant bands, D and G, located at 1309.65 cm−1 and 1587.4 cm−1, respectively.51 The A1g mode of the D band symmetry is due to the vibration mode of sp3 carbon atoms, while the E2g mode of the G band symmetry is due to the vibration mode of sp2 hybridized carbon atoms, directly related to first-order scattering. The position of the D and G bands in the rGO spectrum is shifted compared to the GO spectrum, indicating the successful reduction of rGO.52,53 For rGO/TAPP, three significant peaks are observed at 1323.1 cm−1 (D band), 1518.97 cm−1, and 1599.05 cm−1 (G band) due to the functionalization of TAPP on the surface of rGO. The peak appearing at 1093.54 cm−1 in the TAPP spectrum seems to have disappeared in the successfully synthesized rGO/TAPP composite due to the high porosity and surface-to-volume ratio, which is also clearly depicted in Figs. 5d and 6d. When TAPP interacts with the rGO surface, it may adopt a different conformation compared to its isolated state or π–π* transition of aromatic rings with Q and B bands of TAPP as depicted in the UV–visible spectrum of the rGO/TAPP composite. This change in the molecular arrangement can affect the vibration modes and Raman scattering behavior, leading to a shift in the peak from 1093.54 cm−1 to 1518.57 cm−1. Moreover, when meso-tetra porphyrin interacts with the rGO surface, it may adopt a different orientation or conformation compared to its isolated state. This change in molecular arrangement can affect the vibrational modes and Raman scattering behavior, leading to the disappearance of peaks or shifts in their position.
Atomic Force Microscopy (AFM)
The AFM images (Park XE-7) and histogram of height distribution of GO, rGO, TAPP, and rGO/TAPP in non-contact mode with a 3.0 µm × 3.0 µm scan area are shown in Fig. 5. In gas sensors, surface roughness is one of the most crucial factors which replicate high trapping chances of gas analytes.5 Graphene is a sp2 hybridized material and is expected to be thicker due to oxygen-containing functional groups.53 Although the surface of TAPP is generally smooth, it shows noticeable roughness due to some particle aggregation. The line profiles of height distribution of GO, rGO, TAPP, and rGO/TAPP are also shown in Fig. S1 (Supplementary Material), which was calculated via the Gaussian function of the experimental data. The line profile TAPP is identified throughout a narrow height distribution range. Moreover, TAPP has a smaller surface area than GO, rGO, and rGO/TAPP, with fewer line profile counts. At the same time, the height distribution of rGO is dispersed over a broad range because of its aggregated nature. Also, the functional groups containing oxygen were eliminated; their peaks and valleys are significantly higher than GO. The rGO/TAPP surface area decreased due to the inclusion of TAPP in the rGO matrix. The presence of TAPP causes the accumulation of rGO in rGO/TAPP to disperse while having a relatively low impact on surface roughness, surface area, histogram, and line profile from Table I. The random implantation of rGO nano-leaves with TAPP may be responsible for forming these protrusions. Comparing GO, rGO, TAPP, and rGO/TAPP for surface roughness, TAPP has low surface roughness and rGO possesses high surface roughness. The surface roughness of rGO/TAPP is less than rGO; hence, it will be helpful in recovery time of the sensor (removal of gas molecules from the rGO/TAPP composite matrix).
Field Emission Scanning Electron Microscopy (FESEM)
FESEM images of GO, rGO, TAPP and rGO/TAPP are shown in Fig. 6. The FESEM image of GO (Fig. 6a) shows layered morphology which resembles the reported literature.54 The morphology of rGO (Fig. 6b) shows a highly wrinkled structure as compared to GO due the reduction of oxygen-containing functional groups, which is an indication of successful rGO formation.55 It may be noted that a shortcoming of rGO-based sensors includes high response and recovery times due to the highly wrinkled morphology. The FESEM image of TAPP (Fig. 6c) reveals the short particle assembly. The FESEM image of the rGO/TAPP composite (Fig. 6d) shows successful integration of both TAPP and rGO phases. TAPP particles seem to be integrated on the wrinkled rGO surface. This kind of surface morphology will be useful for sensing applications.
Electrical Characterization
Electrical characterization data were recorded using a Keithley 4200A semiconductor parameter analyzer with a potential range of −5 V to 5 V and 0.05 V step as shown in Fig 7. The I–V measurement for rGO and rGO/TAPP shows s-shaped curves indicating the semiconducting behavior of rGO and rGO/TAPP. rGO is a p-type material, and TAPP is an electron donor. When rGO is combined with TAPP, it causes the generation of electron–hole pairs within the composite.56,57 This transition is reflected by the changes in the resistance, which can be seen in the I–V curves. rGO is shown in a black (solid) line, with a resistance of 115.15 kΩ, and rGO/TAPP is shown in a red (dashed) line with a resistance of 203.92 kΩ.58 An increase in the resistance of the rGO/TAPP composite can be observed, which confirms the successful formation of the rGO/TAPP composite.
Gas Sensing Performance
The dynamic real-time chemiresistive responses of the rGO/TAPP composite were measured using an in-house developed gas sensing system. Two MFCs were used to get the desired concentration of the gas analyte. The fabricated sensor was enclosed in a small air-tight chamber where it was exposed to the desired gas environment. All measurements were recorded using a Keithley 4200A semiconductor parameter analyzer. Nitrogen (N2) gas was used for the baseline, and N2 gas was diluted with the respective gas analyte to get the desired concentration.
The gas sensing performance of the rGO/TAPP composite was investigated with respect to different concentrations of SO2 in the range of 50–200 ppm. In addition, the concentration range of 5–15 ppm was investigated to determine the LOD of the sensor (as 5 ppm is the PEL suggested by OSHA). The results are shown in Fig. 8a and b. When the rGO/TAPP composite was exposed to SO2, the sensor response increased, and for recovery, it was exposed to N2, and the sensor ultimately regained its initial value. This shows that the rGO/TAPP-based sensor behaves like a p-type semiconductor. At 5 ppm of SO2, the rGO/TAPP-based sensor showed response time and recovery time of 57 s and 61 s, respectively (Fig. 8c). The rapid release of SO2 gas analyte from the rGO/TAPP surface shows that this sensor is effective, and the sensor recovery time is only 4 s higher than the response time. These results are highly impactful because the effectiveness of the gas sensor for real-time detection of environmental gaseous pollutants depends on its fast response and recovery time.
(a) Sensing response of rGO/TAPP for a concentration range of 50–200 ppm. (b) Sensing response of rGO/TAPP for a concentration range of 5–15 ppm. (c) Response time and recovery time at 5 ppm. (d) Repeatability at 50 ppm. (e) Reproducibility. (f) Normalized response curves (linearity) with error bar. (g) Stability curve. (h) Selectivity test.
To investigate the repeatability of the rGO/TAPP-based sensor, the sensor was concurrently exposed to three channels of 50 ppm SO2 gas under identical experimental conditions, as shown in Fig. 8d. The starting amplitude of the rGO/TAPP-based sensor remains unchanged after three exposure cycles to SO2 gas. This indicates that the sensor's response to the SO2 exposure cycles did not change much. Sensor reproducibility is one of the most critical factors in getting a highly accurate, commercially viable device with high reliability. Therefore, two more rGO/TAPP chemiresistive sensors were tested at RT with SO2 target gas analyte at concentrations ranging from 50 ppm to 200 ppm. The results shown in Fig. 8e demonstrate the excellent reproducibility of the SO2 gas sensor based on rGO/TAPP. A strong positive correlation between sensor response and SO2 concentration required for the sensor to be used in real-time applications which can be seen in the related fitting equation of the rGO/TAPP sensor as a function of SO2 concentration (X), which can be expressed as Y with a correlation coefficient (R2) of 0.907.
The calibration plot is shown in Fig. 8f. The reproducibility, linearity, and repeatability of the proposed sensor demonstrate its suitability for the real-time detection of environmental gaseous pollutants. Exposure of the rGO/TAPP-based SO2 sensor to 50 ppm SO2 for 40 days at RT confirmed the sensor's long-term stability (Fig. 8g). The performance of the chemiresistive sensor deviated slightly after 20 days but nearly stabilized 40 days after that, indicating strong stiffness.
Moreover, for real-time on-site detection, selectivity is a crucial criterion. Therefore, the rGO/TAPP sensor was investigated at RT in the presence of 50 ppm of various gases, including SO2, CO, NH3, CO2, H2S, and NO2, as shown in Fig. 8h. The SO2 response of the rGO/TAPP-based sensor was significantly higher than other comparable interfering gases, confirming that the rGO/TAPP-based sensor is highly selective to SO2. The rGO/TAPP sensor displayed excellent sensing performance at RT compared to other sensing materials employed in a few previously published SO2 sensors, as indicated in Table II below.
Sensing Mechanism of rGO/TAPP
The probable gas sensing mechanism of rGO/TAPP towards SO2 gas is as shown in the schematic diagram (Fig. 9). rGO is known to be a p-type material, and porphyrins (TAPP) are electron donors. When porphyrin (TAPP) is combined with rGO, TAPP donates electrons to rGO, causing an elevation in the resistance of the rGO/TAPP composite, which was confirmed by the I–V curve (Fig. 7). The electron transition interaction shown in UV–visible spectra shown in Fig. 3c confirms that the region of electrons sharing in the way of S0 to S1, S0 to S2 with π–π* transitions which have been drawn in a schematic diagram of rGO/TAPP sensor (Fig. 9). rGO is a semiconducting material, which is confirmed by energy band gap calculations (Fig. 4b). The energy band gap of rGO is 1.5 eV. Various researchers have already reported that rGO has very little selectivity towards electron acceptor gas, and its response and recovery time is also very high because of the sp2 hybridized structure of rGO and the surface roughness. The line profile of rGO confirmed by AFM image and line profile of rGO are shown in Fig. 5b and Fig. S1(B) (Supplementary Material), respectively. Moreover, rGO has carbon-containing materials, and the distance between two carbon atoms is ~ 0.142 nm. Thus, each carbon atom is connected to the other three carbon atoms. This means that each carbon has one hole because the valence of a single carbon atom is four.
However, when rGO is modified with TAPP, TAPP donates electrons to rGO, thereby increasing the resistance of rGO/TAPP compared to rGO, which was confirmed by the I–V curve (Fig. 7). When SO2 interacts with the surface of the rGO/TAPP composite, electrons must be transferred from the rGO/TAPP to electron-accepting SO2.
As a result, by Eq. 2, positively charged vacancies in the form of holes are generated in rGO/TAPP, thereby increasing the conductivity of the rGO/TAPP composite. This confirms that the sensing mechanism of the rGO/TAPP composite with SO2 gas analyte is based on the electron transfer from rGO/TAPP to adsorbed SO2 on its surface.
Conclusions
The rGO/TAPP porphyrin composite is coated on the pattern of the copper electrode on glass slides to detect SO2 gas analyte. Electron-donating porphyrins (TAPP) interact with rGO, revealing an s-shaped curve in I–V measurement, thereby increasing the resistance value of rGO/TAPP. The rGO/TAPP composite was subjected to various characterizations, including its structural, spectroscopic, morphological, and electrical properties. The performance of the sensor based on the rGO/TAPP composite was evaluated for the detection of SO2 in chemiresistive modality. The rGO/TAPP sensor exhibited highly selective behavior towards the SO2 gas analyte, with linearity in the concentration range of 5–15 ppm and 50–200 ppm. The rGO/TAPP-based sensor showed excellent sensing performance, with a response time of 57 s, recovery time of 61 s at 5 ppm (RT), and LOD of 5 ppm. The proposed sensors also exhibited excellent reproducibility, repeatability, and stability for up to 40 days. This kind of sensor is well suited for real-time detection of hazardous gasses in the environment.
References
F.A. Lagunas-Rangel, W. Liu, and H.B. Schiöth, Can exposure to environmental pollutants be associated with less effective chemotherapy in cancer patients. Int. J. Environ. Res. Public Health 19, 2064 (2022).
N. Ingle, P. Sayyad, M. Deshmukh, G. Bodkhe, M. Mahadik, T. Al-Gahouari, S. Shirsat, and M.D. Shirsat, A chemiresistive gas sensor for sensitive detection of SO2 employing Ni-MOF modified-OH-SWNTs and-OH-MWNTs. Appl. Phys. A. 127, 157 (2021).
H.Y. Mohammed, M.A. Farea, P.W. Sayyad, N.N. Ingle, T. Al-Gahouari, M.M. Mahadik, G.A. Bodkhe, S.M. Shirsat, and M.D. Shirsat, Selective and sensitive chemiresistive sensors based on polyaniline/graphene oxide nanocomposite: a cost-effective approach. J. Sci. Adv. Mater. Devices 7, 100391 (2022).
W. Qi, W. Li, Y. Sun, J. Guo, D. Xie, L. Cai, H. Zhu, L. Xiang, and T. Ren, Influence of low-dimension carbon-based electrodes on the performance of SnO2 nanofiber gas sensors at room temperature. J. Nanotechnol. 30, 345503 (2019).
M.A. Farea, H.Y. Mohammed, P.W. Sayyad, N.N. Ingle, T. Al-Gahouari, M.M. Mahadik, G.A. Bodkhe, S.M. Shirsat, and M.D. Shirsat, Carbon monoxide sensor based on polypyrrole-graphene oxide composite: a cost-effective approach. Appl. Phys. A 127, 681 (2021).
N.L. Myadam, D.Y. Nadargi, J.D. Nadargi, V.R. Kudkyal, F.I. Shaikh, I.S. Mulla, S.S. Suryavanshi, and M.G. Chaskar, Ni/SnO2 xerogels via epoxide chemistry: potential candidate for H2S gas sensing application. J Porous Mater 28, 239 (2021).
M.D. Brown and M.H. Schoenfisch, Electrochemical nitric oxide sensors: principles of design and characterization. Chem. Rev. 119, 11551 (2019).
M. Sun, N. Liu, H. Yu, X. Dong, Y. Yang, and L. Xia, Fabrication of porous WO3/SBA-15 composite NOx gas sensor with a high sensitivity. J Porous Mater 28, 1031 (2021).
E. Pargoletti, F. Tessore, G.D. Carlo, G.L. Chiarello, and G. Cappelletti, Towards low temperature VOCs chemoresistors: graphene oxide versus porphyrin-based materials. Chem. Proc. 5, 60 (2021).
Y.-G. Lee, P.-H. Lee, S.-M. Choi, M.-H. An, and A.-S. Jang, Effects of air pollutants on airway diseases. Int. J. Environ. Res. Public Health 18, 9905 (2021).
N.M. Nurazzi, M.M. Harussani, N.D. Siti-Zulaikha, A.H. Norhana, M. Imran-Syakir, and A. Norli, Composites based on conductive polymer with carbon nanotubes in DMMP gas sensors—an overview. Polimery 66, 85 (2021).
Y. Wang, T. Cheng, C. Lou, X. Liu, X. Li, L. Huang, J. Yao, and X. Liu, Carbon-based light-induced thermoelastic spectroscopy for ammonia gas sensing. Micro Opt. Tech. Lett. 65, 1086 (2023).
H. Qu, L. Huang, Z. Han, Y. Wang, Z. Zhang, Y. Wang, Q. Chang, N. Wei, M.J. Kipper, and J. Tang, A review of graphene-oxide/metal–organic framework composites materials: characteristics, preparation and applications. J. Porous Mater. 28, 1837 (2021).
M. Jahan, Q. Bao, and K.P. Loh, Electrocatalytically active graphene-porphyrin MOF composite for oxygen reduction reaction. J. Am. Chem. Soc. 134, 6707 (2012).
T. Li, W. Yin, S. Gao, Y. Sun, P. Xu, S. Wu, H. Kong, G. Yang, and G. Wei, The combination of two-dimensional nanomaterials with metal oxide nanoparticles for gas sensors: a review. Nanomaterials 12, 982 (2022).
S. Singh, J. Deb, U. Sarkar, and S. Sharma, MoSe2 crystalline nanosheets for room-temperature ammonia sensing. ACS Appl. Nano Mater. 3, 9375 (2020).
D.-B. Moon, A. Bag, H.-B. Lee, M. Meeseepong, D.-H. Lee, and N.-E. Lee, A stretchable, room-temperature operable, chemiresistive gas sensor using nanohybrids of reduced graphene oxide and zinc oxide nanorods. Sens. Actuators B Chem. 345, 130373 (2021).
X. Wang, B. Huang, X. Wu, D. Gu, and X. Li, Enhanced ammonia sensing properties of rGO/WS2 heterojunction based chemiresistive sensor by marginal sulfonate decoration. Sens. Actuators B Chem. 337, 129776 (2021).
Y. Wang, J. Chen, and X. Huang, Adsorption behavior of B-doped/N-doped graphene sheets toward NO2, NO and NH3 molecules: a first-principles study. Phys. Status Solidi C 14, 1600110 (2017).
W. Yuan, A. Liu, L. Huang, C. Li, and G. Shi, High-performance NO2 sensors based on chemically modified graphene. Adv. Mater. 25, 766 (2013).
W. Li, J. Guo, L. Cai, W. Qi, Y. Sun, J.-L. Xu, M. Sun, H. Zhu, L. Xiang, D. Xie, and T. Ren, UV light irradiation enhanced gas sensor selectivity of NO2 and SO2 using rGO functionalized with hollow SnO2 nanofibers. Sens. Actuators B Chem. 290, 443 (2019).
P.W. Sayyad, S.S. Khan, N.N. Ingle, G.A. Bodkhe, T. Al-Gahouari, M.M. Mahadik, S.M. Shirsat, and M.D. Shirsat, Chemiresistive SO2 sensor: graphene oxide (GO) anchored poly (3,4-ethylenedioxythiophene): poly (4styrenesulfonate)(PEDOT: PSS). Appl. Phys. A 126, 857 (2020).
F. Shen, D. Wang, R. Liu, X. Pei, T. Zhang, and J. Jin, Edge-tailored graphene oxide nanosheet-based field effect transistors for fast and reversible electronic detection of sulfur dioxide. Nanoscale 5, 537 (2013).
P.-G. Su and Y.-L. Zheng, Room-temperature ppb-level SO2 gas sensors based on RGO/WO3 and MWCNTs/WO3 nanocomposites. Anal. Methods 13, 782 (2021).
Z. Zhai, X. Zhang, J. Wang, H. Li, Y. Sun, X. Hao, Y. Qin, B. Niu, and C. Li, Washable and flexible gas sensor based on UiO-66-NH2 nanofibers membrane for highly detecting SO2. J. Chem. Eng. 428, 131720 (2022).
M.D. Shirsat, T. Sarkar, J. Kakoullis, N.V. Myung, B. Konnanath, A. Spanias, and A. Mulchandani, Porphyrin-functionalized single-walled carbon nanotube chemiresistive sensor arrays for VOCs. J. Phys. Chem. C 116, 3845 (2012).
A.D. Rushi, K.P. Datta, P.S. Ghosh, A. Mulchandani, and M.D. Shirsat, Selective discrimination among benzene, toluene, and xylene: Probing metalloporphyrin-functionalized single-walled carbon nanotube-based field effect transistors. J. Phys. Chem. C 118, 24034 (2014).
G. Magna, S. Dinc Zor, A. Catini, R. Capuano, F. Basoli, E. Martinelli, R. Paolesse, and C. Di Natale, Surface arrangement dependent selectivity of porphyrins gas sensors. Sens. Actuators B Chem. 251, 524 (2017).
A.D. Rushi, K.P. Datta, P. Ghosh, A. Mulchandani, and M.D. Shirsat, Exercising substituents in porphyrins for real time selective sensing of volatile organic compounds. Sens. Actuators B Chem. 257, 389 (2018).
P. Zhu, F. Song, P. Ma, Y. Wang, C. Chen, and J. Feng, Morphology-controlled self-assembly of a ferrocene-porphyrin based NO2 gas sensor: tuning the semiconducting nature via solvent-solute interaction. J. Mater. Chem. C 4, 10471 (2016).
S.M. Shirsat, C.-H. Chiang, G.A. Bodkhe, M.D. Shirsat, and M.-L. Tsai, High sensitivity carbon monoxide detector using iron tetraphenyl porphyrin functionalized reduced graphene oxide. Discover Nano. 18, 34 (2023).
M. Shaik, V.K. Rao, M. Gupta, K.S.R.C. Murthy, and R. Jain, Chemiresistive gas sensor for the sensitive detection of nitrogen dioxide based on nitrogen doped graphene nanosheets. RSC Adv. 6, 1527 (2016).
A. Siklitskaya, E. Gacka, D. Larowska, M. Mazurkiewicz-Pawlicka, A. Malolepszy, L. Stobiński, B. Marciniak, A. Lewandowska-Andrałojć, and A. Kubas, Lerf–Klinowski-type models of graphene oxide and reduced graphene oxide are robust in analyzing non-covalent functionalization with porphyrins. Sci Rep. 11, 7977 (2021).
M. Sun, C. Zhang, D. Chen, J. Wang, Y. Ji, N. Liang, H. Gao, S. Cheng, and H. Liu, Ultrasensitive and stable all graphene field-effect transistor-based Hg2+ sensor constructed by using different covalently bonded RGO films assembled by different conjugate linking molecules. J. SmartMat. 2, 213 (2021).
H. Lin, X. Cheng, M.-J. Yin, Z. Bao, X. Wei, and B. Gu, Flexible porphyrin doped polymer optical fibers for rapid and remote detection of trace DNT vapor. J. Anal. 145, 5307 (2020).
X. Xu, S. Wang, Y. Yue, and N. Huang, Semiconductive porphyrin-based covalent organic frameworks for sensitive near-infrared detection. ACS Appl. Mater. Interfaces 12, 37427 (2020).
H.C. Su, T.-T. Tran, W. Bosze, and N.V. Myung, Chemiresistive sensor arrays for detection of air pollutants based on carbon nanotubes functionalized with porphyrin and phthalocyanine derivatives. Sens. Actuators Rep. 2, 100011 (2020).
D.C. Marcano, D.V. Kosynkin, J.M. Berlin, A. Sinitskii, Z. Sun, A. Slesarev, L.B. Alemany, W. Lu, and J.M. Tour, Improved synthesis of graphene oxide. ACS Nano 4, 4806 (2010).
A.T. Smith, A.M. LaChance, S. Zeng, B. Liu, and L. Sun, Synthesis, properties, and applications of graphene oxide/reduced graphene oxide and their nanocomposites. Nano Mater. Sci. 1, 31 (2019).
S.N. Alam, N. Sharma, and L. Kumar, Synthesis of graphene oxide (GO) by modified Hummers method and its thermal reduction to obtain reduced graphene oxide (rGO). Graphene 06, 1 (2017).
A.T. Habte and D.W. Ayele, Synthesis and characterization of reduced graphene oxide (rGO) started from graphene oxide (GO) using the tour method with different parameters. Adv. Mater. Sci. Eng. 2019, 1 (2019).
Y. Liu, B. Sajjadi, W.-Y. Chen, and R. Chatterjee, Ultrasound-assisted amine functionalized graphene oxide for enhanced CO2 adsorption. Fuel 247, 10 (2019).
M. Managa, S. Mgidlana, S. Khene, and T. Nyokong, Optical limiting properties of indium 5, 10, 15, 20-tetrakis (4-aminophenyl) porphyrin covalently linked to semiconductor quantum dots. Inorg. Chim. Acta 511, 119838 (2020).
T. AL-Gahouari, P. Sayyad, N. Ingle, M. Mahadik, M. Farea, H. Mohammed, S. Shirsat, and M. Shirsat, Resolution improvement for anodic stripping signals of lead and detached indium from reduced graphene oxide/indium tin oxide (rGO/ITO) electrode using bromide ion. Appl. Phys. A. 127, 326 (2021).
N.M.S. Hidayah, W.-W. Liu, C.-W. Lai, N.Z. Noriman, C.-S. Khe, U. Hashim, and H.C. Lee, Comparison on graphite, graphene oxide and reduced graphene oxide: synthesis and characterization. AIP Conf. Proc. 1892, 150002 (2017).
L. Giovanelli, H.-L. Lee, C. Lacaze-Dufaure, M. Koudia, S. Clair, Y.-P. Lin, Y. Ksari, J.-M. Themlin, M. Abel, and A.A. Cafolla, Electronic structure of tetra (4-aminophenyl) porphyrin studied by photoemission, UV–Vis spectroscopy and density functional theory. J. Electron. Spectrosc. Relat. Phenom. 218, 40 (2017).
D. Ma, B. Li, Z. Cui, K. Liu, C. Chen, G. Li, J. Hua, B. Ma, Z. Shi, and S. Feng, Multifunctional luminescent porous organic polymer for selectively detecting iron ions and 1, 4-dioxane via luminescent turn-off and turn-on sensing. ACS Appl. Mater. Interfaces 8, 24097 (2016).
G. Zhang, A.F.M. EL-Mahdy, L.R. Ahmed, B.M. Matsagar, S. Al-Saeedi, S.-W. Kuo, and K.C.-W. Wu, Metal complexes of the porphyrin-functionalized polybenzoxazine. J. Polym. 14, 449 (2022).
I. Osica, G. Imamura, K. Shiba, Q. Ji, L.K. Shrestha, J.P. Hill, K.J. Kurzydłowski, G. Yoshikawa, and K. Ariga, Highly networked capsular silica-porphyrin hybrid nanostructures as efficient materials for acetone vapor sensing. ACS Appl. Mater. Interfaces. 9, 9945 (2017).
A. Maimaiti, R. Abdurahman, N. Kari, Q.-R. Ma, K. Wumaier, P. Nizamidin, S. Abliz, and A. Yimit, Highly sensitive optical waveguide sensor for SO2 and H2S detection in the parts-per-trillion regime using tetraaminophenyl porphyrin. J. Mod. Opt. 67, 507 (2020).
N. Sharma, V. Sharma, Y. Jain, M. Kumari, R. Gupta, S.K. Sharma, and K. Sachdev, Synthesis and characterization of graphene oxide (GO) and reduced graphene oxide (rGO) for gas sensing application. Macromol. Symp. 376, 1700006 (2017).
L. Guo, Y.-W. Hao, P.-L. Li, J.-F. Song, R.-Z. Yang, X.-Y. Fu, S.-Y. Xie, J. Zhao, and Y.-L. Zhang, Improved NO2 gas sensing properties of graphene oxide reduced by two-beam-laser interference. Sci Rep. 8, 4918 (2018).
P. Ranjan, P. Tiwary, A.K. Chakraborty, R. Mahapatra, and A.D. Thakur, Graphene oxide based free-standing films for humidity and hydrogen peroxide sensing. J Mater. Sci. Mater. Electron. 29, 15946 (2018).
M.S. Eluyemi, M.A. Eleruja, A.V. Adedeji, B. Olofinjana, O. Fasakin, O.O. Akinwunmi, O.O. Ilori, A.T. Famojuro, S.A. Ayinde, and E.O.B. Ajayi, Synthesis and characterization of graphene oxide and reduced graphene oxide thin films deposited by spray pyrolysis method. Graphene 05, 143 (2016).
S. Drewniak, R. Muzyka, A. Stolarczyk, T. Pustelny, M. Kotyczka-Morańska, and M. Setkiewicz, Studies of reduced graphene oxide and graphite oxide in the aspect of their possible application in gas sensors. Sensors 16, 103 (2016).
A. Lipatov, A. Varezhnikov, P. Wilson, V. Sysoev, A. Kolmakov, and A. Sinitskii, Highly selective gas sensor arrays based on thermally reduced graphene oxide. Nanoscale 5, 5426 (2013).
S.M. Shirsat, G.A. Bodkhe, M.M. Sonawane, B.W. Gawali, and M.D. Shirsat, Multivariate analysis of a cobalt octaethyl porphyrin-functionalized SWNT microsensor device for selective and simultaneous detection of multiple analytes. J. Electron. Mater. 50, 5780 (2021).
Z. Bo, X. Shuai, S. Mao, H. Yang, J. Qian, J. Chen, J. Yan, and K. Cen, Green preparation of reduced graphene oxide for sensing and energy storage applications. Sci. Rep. 4, 4684 (2014).
R. Kumar, D.K. Avasthi, and A. Kaur, Fabrication of chemiresistive gas sensors based on multistep reduced graphene oxide for low parts per million monitoring of sulfur dioxide at room temperature. Sens. Actuators B Chem. 242, 461 (2017).
D. Zhang, J. Liu, C. Jiang, P. Li, and Y. Sun, High-performance sulfur dioxide sensing properties of layer-by-layer self-assembled titania-modified graphene hybrid nanocomposite. Sens. Actuators B Chem. 245, 560 (2017).
V. Dhingra, S. Kumar, R. Kumar, A. Garg, and A. Chowdhuri, Room temperature SO2 and H2 gas sensing using hydrothermally grown GO-ZnO nanorod composite films. Mater. Res. Express. 7, 065012 (2020).
Acknowledgments
The authors sincerely thank DST-SERB, New Delhi (Project No. E.E.Q./2017/000645), DST-FIST (Project No. SR/FST/PSI-210/2016(C) dtd. 16/12/2016), UGC-SAP Programme (F.530/16/DRS-I/2016 (SAP-II) Dt.16-04-2016), Rashtriya Uchachatar Shiksha Abhiyan (RUSA), Government of Maharashtra, UGC—DAE CSR (RRCAT), Indore (Project No. CSR-IC-BL66/CRS-183/2016-17/847), and Inter-University Accelerator Centre (IUAC), New Delhi, India (UFR no. 62320), for providing financial support. Dr. Vijaykiran N. Narwade acknowledges the UGC DS Kothari post-doctoral fellowship for financial assistance. Mahendra D. Shirsat gratefully acknowledges the Slovak Academic Information Agency (SAIA) and Department of Nuclear Physics and Biophysics, Faculty of Mathematics, Physics, and Informatics, Comenius University, Bratislava, Slovak Republic, for the sanction of scholarship under the framework of National Scholarship Program (NSP) of Slovak Republic.
Funding
Open access funding provided by The Ministry of Education, Science, Research and Sport of the Slovak Republic in cooperation with Centre for Scientific and Technical Information of the Slovak Republic.
Author information
Authors and Affiliations
Contributions
KAS—Experimental work, data analysis with the drafting of the manuscript ; VP and RB—Experimental work, data analysis; VNN—Planning, conceptualization, editing of the manuscript; BND—Manuscript editing; NNI—Data analysis; M-LT: Data analysis and manuscript editing; TH—Conceptualization and manuscript editing, MDS—Conceptualization, planning of Experiments, Data Analysis, drafting and editing of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Khune, A.S., Padghan, V., Bongane, R. et al. Highly Selective Chemiresistive SO2 Sensor Based on a Reduced Graphene Oxide/Porphyrin (rGO/TAPP) Composite. J. Electron. Mater. 52, 8108–8123 (2023). https://doi.org/10.1007/s11664-023-10711-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11664-023-10711-4