Abstract
The present study focuses on a specific step of the metallurgical path of purification to provide solar-grade silicon: the removal of boron through the injection of H2O(g)-H2(g)-Ar(g) (cold gas process) or of Ar-H2-O2 plasma (plasma process) on stirred liquid silicon. We propose a way to predict silicon and boron flows from the liquid silicon surface by using a CFD model (©Ansys Fluent) combined with some results on one-dimensional diffusive-reactive models to consider the formation of silica aerosols in a layer above the liquid silicon. The comparison of the model with experimental results on cold gas processes provided satisfying results for cases with low and high concentrations of oxidants. This confirms that the choices of thermodynamic data of HBO(g) and the activity coefficient of boron in liquid silicon are suitable and that the hypotheses regarding similar diffusion mechanisms at the surface for HBO(g) and SiO(g) are appropriate. The reasons for similar diffusion mechanisms need further enquiry. We also studied the effect of pressure and geometric variations in the cold gas process. For some cases with high injection flows, the model slightly overestimates the boron extraction rate, and the overestimation increases with increasing injection flow. A single plasma experiment from SIMaP (France) was modeled, and the model results fit the experimental data on purification if we suppose that aerosols form, but it is not enough to draw conclusions about the formation of aerosols for plasma experiments.

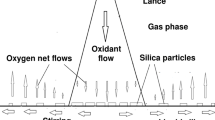
Source: Reproduced with permission from Sortland[3]


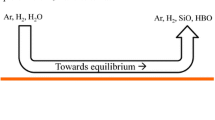
Source: Reproduced with permission from Altenberend[4]








Similar content being viewed by others
Notes
In Appendix A, we show that the hypothetical appearance of Si(OH)4 should not affect the flow of silicon and oxygen toward the surface.
In reality, the concentration of H2O(g) is probably lower near the surface than at injection because Ar and H2O(g) have similar diffusivities and some of the H2O(g) has already reacted.
1687K is the fusion temperature of silicon according to the SGTE-SGPS database.[28]
Abbreviations
- \(D_{\text {X}}\) :
-
Diffusivity of species X
- \(P_{\text {X}}\) :
-
Partial pressure of species X
- \(p_{\text {X}}\) :
-
Dimensionless partial pressure of species X, taken as the value when expressed in bar
- \(\Psi _{\text {X}}\) :
-
\(D_{\text {X}}/D_{\text {O}_2}\)
- \(J_{\text {X}}\) :
-
Flow of species or atom X in mol/m2/s
- \(P_{\text {O}_2}^{\text {eff}} = \frac{1}{2}\psi _{\text {H}_2\text {O}}P_{\text {H}_2\text {O}} +\frac{1}{2}\psi _{\text {SiO}}P_{\text {SiO}} + \psi _{\text {SiO}_2}P_{\text {SiO}_2}\) :
-
Effective pressure for oxygen
- \(P_{\text {Si}}^{\text {eff}}=\psi _{\text {SiO}}P_{\text {SiO}} + \psi _{\text {SiO}_2}P_{\text {SiO}_2}\) :
-
Effective pressure for Si
- \(P_{\text {O}_2}^{\text {eff,surf}}\) :
-
Effective pressure for oxygen at the surface
- \(P_{\text {O}_2}^{\text {eff,ext}}\) :
-
Effective pressure for oxygen at injection
- \(P_{\text {Si}}^{\text {sat}}\) :
-
Saturation pressure of Si(g), which is also the pressure of Si(g) at the surface
- \(\delta \) :
-
Thickness of the boundary layer
- c:
-
Total molar concentration of gas
- \(c_B\) :
-
Concentration of boron in the fluid
- \(x_B\) :
-
Mole fraction of boron in liquid
- \(K_{\text {nuc}}\) :
-
Equilibrium constant of the reaction SiO(g) + H2O(g) \(\leftrightarrows \) SiO2(s/l) + H2(g)
- R :
-
Universal gas constant
- Rf:
-
Enrichment factor
- \(k_{\rm{t}}\) :
-
Total mass transfer coefficient
References
J. Safarian, G. Tranell, and M. Tangstad, Energy Procedia, vol. 20, pp. 88–97, 2012.
Y. Delannoy, M. Heuer, E. Øvrelid, and S. Pizzini, 3 Conventional and Advanced Purification Processes of MG Silicon. CRC Press, Boca Raton (2017).
Ø.S. Sortland: Ph.D. Thesis, NTNU, Norway, 2015.
J. Altenberend: Ph.D. Thesis, Grenoble-INP, France, 2012.
Ø. S. Sortland and M. Tangstad, Metallurgical and Materials Transactions E, vol. 1E, pp. 211–225, 2014.
J. Altenberend, G. Chichignoud, and Y. Delannoy, Metallurgical and Materials Transactions E, vol. 4E, pp. 41–50, 2017.
M. Vadon, Y. Delannoy, and G. Chichignoud, Metallurgical and Materials Transactions B, vol 48, pp. 1667–1674.
ANSYS Fluent, 14.5 Theory Guide. ANSYS Inc., Canonsburg, PA, 2012.
S.B. Pope: Combust. Theor. Model., vol. 1(1), 1997. https://www.tandfonline.com/doi/abs/10.1080/713665229.
Y. Delannoy, C. Alemany, K.-I. Li, P. Proulx, and C. Trassy, Solar Energy Mater. Solar Cells, vol. 72, no. 1, pp. 69–75, 2002.
D. Pelletier, Y. Delannoy, and C. Trassy: High Temp. Mater. Process., vol. 14, no. 3, 211–221, 2010.
M. Majchrzak, J. Altenberend, Y. Delannoy, and G. Chichignoud: Bull. Am. Physi. Soc., vol. 55, 2010.
D. Pelletier: Ph.D. Thesis, 2006.
J.-G. Lacombe: Ph.D. Thesis, Thèse INP Grenoble, 2008.
J. Degoulange: Ph.D. Thesis, Institut National Polytechnique de Grenoble-INPG, 2008.
C. Alemany, C. Trassy, B. Pateyron, K.-I. Li, and Y. Delannoy, Solar energy materials and solar cells, vol. 72, no. 1, pp. 41–48, 2002.
M.W. Chase: JANAF Thermochemical Tables, by Chase, MW, vol. 1, American Chemical Society, Washington, DC, 1986.
M. Page, ‘J. Phys. Chem., vol. 93, no. 9, pp. 3639–3643, 1989.
S. Gorokhov, L.N. Polisadin, and A. Emelyanov: XII All Union Conference on Calorimetry and Chemical Thermodynamics,1991,Krasnoyarsk,Theses of the Reports, No. 1, p. 58, 1991.
N. S. Jacobson and D. L. Myers, J. Phys. Chem. B, vol. 115, no. 45, pp. 13253–13260, 2011.
L.V.G.I.V.V.C.B. Alcock: Thermodynamic Properties of Individual Substances. Vol. 3, Elements B, Al, Ga, In, Tl, Be, Mg, Ca, Sr, Ba and Their Compunds, Part 1, Methods and Computation. Boca Raton : CRC Press, 1994.
I. Ansara, A. Dinsdale, and M. Rand: Office for Official Publications of the European Communities, 1998.
R.A. Svehla: Estimated Viscosities and Thermal Conductivities of Gases at High Temperatures, Tech. Report, National Aeronautics and Space Administration, Lewis Research Center, Cleveland, 1962.
S. Schnurre, J. Gröbner, and R. Schmid-Fetzer, Journal of Non-Crystalline Solids, vol. 336, no. 1, pp. 1–25, 2004.
M. Ratto, E. Ricci, E. Arato, and P. Costa, Metallurgical and Materials Transactions B, vol. 32, no. 5, pp. 903–11, 2001.
A. V. Plyasunov, Geochimica et Cosmochimica Acta, vol. 75, no. 13, pp. 3853–3865, 2011.
E. Opila, Calphad, vol. 55, pp. 32–40, 2016.
C. Bale, P. Chartrand, S. Degterov, G. Eriksson, K. Hack, R. B. Mahfoud, J. Melançon, A. Pelton, and S. Petersen, Calphad, vol. 26, no. 2, pp. 189–228, 2002.
R. Bird, W. Stewart, and E. Lightfoot, Transport Phenomena. Wiley International edition, Wiley, 2007.
ANSYS Fluent, 14.0 User’s Guide. ANSYS Inc., 2012.
D. Baulch, C. Bowman, C. Cobos, R. Cox, T. Just, J. Kerr, M. Pilling, D. Stocker, J. Troe, W. Tsang, et al.: Journal of physical and chemical reference data, vol. 34, no. 3, pp. 757–1397, 2005.
C. Park, Journal of Thermophysics and Heat Transfer, vol. 3, no. 3, pp. 233–244, 1989.
J. Park, E. Pfender, and C. Chang, Plasma Chemistry and Plasma Processing, vol. 20, no. 2, pp. 165–181, 2000.
M. L Rightley and F. Williams, Combustion Science and Technology, vol. 125, no. 1-6, pp. 181–200, 1997.
P. Saxena and F. A. Williams, Combustion and Flame, vol. 145, no. 1, pp. 316–323, 2006.
A. A. Konnov, Combustion and flame, vol. 152, no. 4, pp. 507–528, 2008.
Acknowledgments
We thank the Rhone-Alpes region for the CMIRA program and Elisabeth Blanquet from SIMAP-TOP.
Author information
Authors and Affiliations
Corresponding author
Additional information
Manuscript submitted August 24, 2017.
Appendices
Appendix A: Simplified 1D Isothermal Model
In this section, we use the one-dimensional model by Vadon et al.[7] to reason why the silicon outflow in diluted cases is half of what it would be if the nucleation of aerosols did not occur.
We consider a stagnant boundary layer of thickness \(\delta \), where the only reactive species are H2O (\(P_{\text {H}_2\text {O}}^0\)) and H2(g). Liquid silicon is at the reactive surface (z = 0). As represented by Ratto[25] and Vadon et al.,[7] the boundary layer is made of a lower homogeneous sublayer (i.e., without silica aerosols), an intermediate heterogeneous sublayer (equilibrium \(K_{nuc}\) of the reaction SiO(g) + H2O(g) \(\leftrightarrows \) SiO2(s/l) + H2(g)) and a homogeneous upper sublayer.
Hypotheses:
-
Thermodynamic equilibrium everywhere.
-
Net flow of oxygen and hydrogen atoms at the surface equals zero.
For this part, we define the effective pressures, which are used to express the flow of oxygen and silicon atoms as parts of gas molecules:
Vadon et al. show that because of the formation of silica aerosols in every point of the layer, the quantity per time unit of oxygen atoms going from the gas phase to the silica phase is twice the quantity per unit time of silicon atoms going from the gas phase to the silica phase. As a result (index 0 means at injection):
Thus, by looking at the conditions at the surface (z = 0) and at the top of the boundary layer (\(z\,=\,\delta \)), the molar flow per m\(^2\) (\(J_{\text {Si}}\)), and using the hypothesis of a zero net oxygen flow from the surface:
Then, using thermodynamic data from JANAF,[17] we can neglect \(\Psi _{\text {Si}}P_{\text {Si}}^{\text {sat}}\) and \( \Psi _{\text {H}_2\text {O}}P_{\text {H}_2\text {O}}^{\text {surf}}\). They are small compared with \(P_{\text {H}_2\text {O}}^0\), which is higher than 100 Pa in the studied cases.
Using data from Plyasunov et al.[26] (also cited in Opila et al.[27]) for Si(OH)4, we can also neglect \(P_{\text {Si(OH)}_4}\) in compararison with \(P_{\text {SiO}}\) at the surface in the presence of liquid silicon. At thermodynamic equilibrium, in the presence of Si(l), \(\frac{p_{\text {SiO}}^4 p_{\text {H}_2}^2}{p_{\text {Si(OH)}_4}} > 3 \times 10^6\) for \(T > {1687}\,{\text{K}}\)Footnote 4 when pressures are expressed in bars. Thermodynamic data for Si(OH)\(_4\) is presented in Table VII. In case the values of \(P_{\text {Si(OH)}_4}\) became higher compared with \(P_{\text {SiO}}\) in other zones upwards of the boundary layer, this would be of no impact on the inflow of oxygen toward the surface and on the outflow of silicon.
This enables us to build a simplified model where only the species Si(l), SiO(g) and H\(_2\)O(g) are taken into account. By using the conditions at the intersection of the homogeneous sublayer (zero flow of oxygen atoms) and the heterogeneous sublayer, like in Vadon et al.,[7] we can thus find an analytical expression of the partial pressure of SiO(g) at the surface in Eq. [5].
Appendix B: Dependence of Ratios of Diffusivities on Temperature
In Bird et al.,[29] the following equations are given for the binary diffusivity of i in j:
At the high temperatures of the process, above 1000 K, we can make the following approximation:
Hence \(D_{ij}\) is approximatively proportional to \(T^{3/2+0.15610}\), and the ratio of two different diffusivities has a dependence on temperature that can be neglected.
Appendix C: Simplified Non-isothermal Model: Diluted Case
In this part, we provide arguments to confirm the relationship [6] in the non-isothermal case with the injection of cold gases. As shown in Appendix B, the diffusivity ratios do not vary significantly with temperature. In the diluted case, we write Fick’s law for gases for a specie X:
The linear relationship [19] due to the appearance of silica in the diluted case then becomes:
We then obtain the relationship:
In the case without aerosols, we would have:
We then find the relationship [4] again. The negative temperature gradient in cold gas does however modify the equilibrium constant, favoring the formation of silica aerosols and diminishing the partial pressures of reactive species SiO(g) and H\(_2\)O(g) relative to the isothermal case. This will diminish \(P_{\text {SiO}}^{\text {surf}}\) relative to the isothermal case, thus strengthening the approximation of Eq. [6] relative to the isothermal case.
In the present model, we did not account for the effect of thermodiffusion. The thermodiffusion effect increases the diffusion rate of lighter molecules toward the heated surface compared with heavier molecules.[30] In the cold gas case, this would lead SiO(g) [which is heavier than H\(_2\)O(g), H\(_2\)(g) and Ar(g)] to diffuse more rapidly from the heated surface, which would lower the concentration of SiO(g) at the surface relative to the present model. Hence, this makes approximation [4] in which the formation of silica aerosol reduces the outflow of silicon by a factor of two even more accurate.
Rights and permissions
About this article
Cite this article
Vadon, M., Sortland, Ø., Nuta, I. et al. CFD Modeling of Boron Removal from Liquid Silicon with Cold Gases and Plasma. Metall Mater Trans B 49, 1288–1301 (2018). https://doi.org/10.1007/s11663-018-1228-5
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11663-018-1228-5