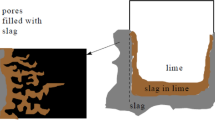
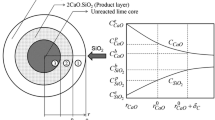
Abstract
A modified model for prediction of flux dissolution in oxygen steelmaking process is presented in this study. The aim of this paper is to introduce a procedure for simulating the amount of dissolved lime with respect to the saturation concentration of CaO by coupling the existing thermodynamic and kinetic models simultaneously. The procedure is developed to calculate the saturation concentrations/solubility of CaO in slag using thermodynamic models namely FactSage™, Cell Model, and Thermo-Calc™. Total amount of dissolved lime is evaluated by integrating solubility values in the rate equation of lime dissolution over time taking into account the effects of physical properties and temperature of slag and particle size of flux additions and validated against industrial data available in literature. Comparison between measured and calculated undissolved lime shows a good agreement between them using any thermodynamic models even though there are some differences in the predictions of saturation concentration of CaO in slag. It has been shown that two distinct control mechanisms for lime dissolution in BOF slags exist and consideration of the free lime-controlled mechanism is essential for accurate prediction of dissolution rate of lime in slag.











Similar content being viewed by others
Abbreviations
- W L :
-
Mass of dissolved lime (kg)
- ρ L :
-
Density of lime (kg/m3)
- n L :
-
Number of lime particles
- S :
-
Surface area of particle (m2)
- t i :
-
Time (min)
- dr/dt :
-
Decrease radius of lime particles due to dissolution during a given time step (m/min)
- Re :
-
Reynold’s number (dimensionless)
- Sc:
-
Schmidt number (dimensionless)
- Sh:
-
Sherwood number (dimensionless)
- u p :
-
Settling velocity of particles (m/s)
- d p :
-
Diameter of solid particle (m)
- μ s :
-
Viscosity of slag (kg/m s)
- ρ s :
-
Density of slag(kg/m3)
- β :
-
Modifying factor for settling velocity of flux particle (dimensionless)
- t :
-
Weight/capacity of LD converter, Metric tonnes
- R k ii and R k ij :
-
Number of symmetric (i–k–i) and asymmetric (i–k–j) cells, respectively
- v ik :
-
Anion stoichiometric index of the component
- x ik :
-
Mole fraction of cation-i and anion-k
- m, p :
-
Number of cations and anions, respectively
- W ij :
-
Formation energy of an asymmetric cell
- E ij :
-
Interaction energy between symmetric and asymmetric cells
- G M :
-
Free energy of mixing
- Ω:
-
The partition coefficient
- a i :
-
Activity of a slag component
References
Y. Satyoko and W E. Lee: Br. Ceram. Trans., 1999, vol. 98, pp. 261–65.
T. Hamano, M. Horibe, and K. Ito: ISIJ Int., 2004, vol. 44, pp. 263–67.
T. Hamano, S. Fukagai, and F. Tsukihashi: ISIJ Int., 2006, vol. 46, pp. 490–95.
J. Liu, M. Guo, P. T. Jones, F. Verhaeghe, B. Blanpain, and P. Wollants: J. Eur. Ceram. Soc., 2007, vol. 27, pp. 1961–72.
S. H. Amini, M P. Brungs, S. Jahanshahi, and O. Ostrovski: Metall. Mater. Trans. B, 2006, vol. 37, pp. 773–80.
S.H. Amini, M. Brungs, S. Jahanshahi, and O. Ostrovski: ISIJ Int., 2006, vol. 46, pp. 1554–59.
S.Haji Amini, O. Ostrovski, and M P. Brungs: VII Int. Conf. Molten Slags Fluxes Salts, 2004, pp. 595–600.
M. Matsushima, S. Yadoomaru, K. Mori, and Y. Kawai: Trans. ISIJ, 1977, vol. 17, pp. 442–49.
H. Y. Sohn: Metall. Trans. B, 1978, vol. 9, pp. 89–96.
N. Maruoka, A. Ishikawa, H. Shibata, and S. Kitamura: High Temp. Mater. Process., 2013, vol. 32, pp. 15–24.
T. Deng: Ph.D Thesis, Royal Institute of Technology (KTH), 2012.
S. Kitamura, H. Shibata, and N. Maruoka: Steel Res. Int., 2008, vol. 79, p. 586.
I. Muchi, S. Asai, and M. Miwa: in Int. Conf. Sci. Technol. Iron Steel, Tokyo, 1970, pp. 347–51.
N. Dogan, G.A. Brooks, and M A. Rhamdhani: ISIJ Int., 2009, vol. 49, pp. 1474–82.
E. Graveland-Gisolf, P. Mink, A. B. Snoeijer, E. Barker, R. Boom, D. Dixit, and B. Deo: Steel Res., 2003, vol. 74, pp. 125–30.
Y. Lytvynyuk, J. Schenk, M. Hiebler, and A. Sormann: Steel Res. Int., 2014, vol. 85, pp. 537–43.
F. Oeters: Metallurgy of Steelmaking, Verlag Stahleisen GmbH, Dusseldorf, 1994.
B.J. Keene and Mills K.C.: Density of Molten Slags, in Slag Atlas, Verlag Stahleisen GmbH, Dusseldorf, 1995.
R Clift, J.R Grace, and M.E Weber: Bubbles, Drops and Particles, 1st ed., New York, 1978.
C. Cicutti, M. Valdez, T. Pérez, R. Donayo, and J. Petroni: Lat. Am. Appl. Res., 2002, vol. 32, pp. 237–40.
M. Umokoshi, M. Katsumi, and Y. Kawai: Trans. ISIJ, 1984, vol. 24, pp. 532–39.
C. Cicutti, M. Valdez, T. Perez, J. Petroni, A. Gomez, R. Donayo, and L. Ferro: in 6th Int. Conf. Molten Slags, Fluxes Salts, Stockholm-Helsinki, 2000, p. 367.
M.S. Millman, A. Overbosch, A. Kapilashrami, D. Malmberg, and M. Brämming: Ironmak. Steelmak., 2011, vol. 38, pp. 499–509.
M.S. Millman, A. Kapilashrami, M. Bramming, and D. Malmberg: Imphos: Improving Phosphorus Refining. European commission report-Research fund for coal and steel unit, Luxemborg, 2011.
I H. Jung, S A. Decterov, and A D. Pelton: J. Eur. Ceram. Soc., 2005, vol. 25, pp. 313–33.
Y.B. Kang and I H Jung: in Int. Symp. Molten Salts Slags, Santiago, 2009.
S A. Decterov, I-H. Jung, and A D. Pelton: J. Am. Ceram. Soc., 2004, vol. 85, pp. 2903–10.
M.L Kapoor and M.G Frohberg: in Chem. Metall. Iron Steel, Iron and Steel Institute, London, 1973, pp. pp. 17–22.
H. Gaye and J.Welfringer: in 2nd Int. Symp. Metall. Slags Fluxes, Metallurgical Society of AIME, Warrendale, PA, 1984, p. 357.
K. Graham: Ph.D Thesis, McMaster University, 2008.
H. Gaye, J Lehmann, T. Matsumiya, and W. Yamada: in 4th Int. Conf. Molten Slags Fluxes, 1992, pp. 103–08.
H. Lehmann, J., and Gaye: Rev. Int. Des Hautes Temp. Des Refract., 1992, vol. 28, pp. 81–90.
Y. Chen, G. Brooks, and S.Nightingale: Can. Metall. Q., 2005, vol. 44, pp. 323–30.
A. Masui, K. Yamada, and K. Takahashi: in Role Slag Basic Oxyg. Steelmak. Process. McMaster Symp. Iron Steelmak. No. 4, 1976, pp. 3.1–3.28.
S. Asai and I. Muchi: Trans. ISIJ, 1970, vol. 10, pp. 250–63.
F. Bardenheuer, H. vom Ende, and K.G. Speith: Blast Furn. Steel Plant, 1970, vol. 58, pp. 401–6.
P. Williams, M. Sunderland, and G. Briggs: Ironmak. Steelmak., 1982, vol. 9, pp. 150–62.
Acknowledgments
The financial support by the Steel Research Center, McMaster University is gratefully acknowledged by the authors. The authors also wish to thank Mr.Alan Galindo for his help with regard to the Cell Model calculations.
Author information
Authors and Affiliations
Corresponding author
Additional information
Manuscript submitted January 20, 2016.
Appendix A: Calculation Procedure of Saturation Concentration of CaO Using Cell Model[32]
Appendix A: Calculation Procedure of Saturation Concentration of CaO Using Cell Model[32]
Cell Fraction Determination
In this step, for a given slag composition, the combination of cells (R k ii and R k ij ) is determined such that the partition coefficient is maximized and hence yields the minimum value of free energy while satisfying the mass balance equations.
Calculation of Free Energy
The free energy of liquid slag may be approximated from the partition function as
where G M is the free energy of mixing, R is the ideal gas constant, T is the temperature, E form is the energy necessary for the formation of asymmetric cells from symmetric ones, E int is the parameter introduced to describe the energy of interaction between different cells, P the total number of possible permutations of anions and cations, U is the total number of randomly distinguishable permutations, and U* is the maximum number of randomly distinguishable permutations. The partition coefficient Ω, in the above equation is a measure of the number of states accessible to the system at a given temperature was defined as
where g i is the degeneracy factor and refers to the number of states having energy e i . The above equation is simplified by considering the ‘most probable’ energetic arrangement that maximizes the partition function
Component Activity Determination
Component activities referred to their pure liquid standard states are determined by computing the partial molar free energy of mixing for each slag component as
where δij is Kronecker’s symbol (δij = 1 for i = j and δij = 0 for i ≠ j).
Calculation of Saturation Concentration
The saturation of CaO is calculated by iteratively calculating the activities of a system at different compositions until one of the stoichiometric components had precipitated as a solid phase. At initial upper and lower saturation points for a component (WThigh = 100 wt pct; WTlow = 0 wt pct), the cell fractions (R k ij ), free energy (G M), and slag activities were recursively calculated until the upper and lower bounds have converged to within 0.01 wt pct. CaO saturation was assumed at a component activity (solid reference) of 0.9995 in order to ensure algorithm convergence at the true saturation point.
Rights and permissions
About this article
Cite this article
Kadrolkar, A., Andersson, N.Å.I. & Dogan, N. A Dynamic Flux Dissolution Model for Oxygen Steelmaking. Metall Mater Trans B 48, 99–112 (2017). https://doi.org/10.1007/s11663-016-0777-8
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11663-016-0777-8