Abstract
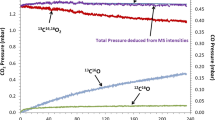
The oxidation behavior of 5 wt pct Cr (5Cr) ferritic steel in air at high temperatures has been investigated. A connected Cu-rich layer adjacently forms in inner oxidation layer (IOL) at 500 °C to 600 °C in addition to the formation of a continuous Cr-rich and Si-rich ribbon. Their synergistic effects imped growth of the Fe-rich oxide film towards the outer oxidation layer (OOL) and thus improve the oxidation resistance of 5Cr steel. With increasing oxidation temperature, the Cr, Si, and Cu oxide scales are gradually damaged and spallation of the oxide scale occurs at 1000 °C. Regarding the reaction kinetics, oxidation is controlled by oxygen inward diffusion. A gas–solid model is used to describe the oxidation behavior of 5Cr steel at 500 °C to 900 °C and there is good agreement with the experimental data. In particular, this model can predict the oxidation behavior and the typical results at 750 °C and 850 °C are provided.









Similar content being viewed by others
References
M. Cortie: Ferritic stainless steels, in: Encyclopedia of Materials: Science and Technology, 2nd ed., Elsevier, Oxford, 2001, pp. 3037-9.
V.B. Trindade, R. Borin, B.Z. Hanjari, S. Yang, U. Krupp, and H. Christ: Mater. Res., 2005, vol. 8, pp. 365-69.
A.R. Setiawan, M.H.B. Ani, M. Ueda, K. Kawamura, and T. Maruyama: ISIJ Int., 2010, vol. 50, pp. 259-63.
I.B. Singh, O.P. Modi, and G. Ruhi: J. Sol-Gel Sci. Technol., 2015, vol. 74, pp. 685-91.
A.S. Khanna and J.B. Gnanamoorthy: Oxid. Met., 1982, vol. 18, pp. 315-30.
P.A. Labun, J. Covington, K. Kuroda, G. Welsch, and T.E. Mitchell: Metall. Mater. Trans. A, 1982, vol. 13, pp. 2103-12.
A.S. Khanna, B.B. Jha, and B. Raj: Oxid. Met., 1985, vol. 23, pp. 159-76.
J. Wang, S. Lu, L. Rong, D. Li, and Y. Li, Corros. Sci., 2016, vol. 111, pp. 13-25.
G.O. Lloyd, S.R.J. Saunders, B. Kent, and A. Fursey: Corros. Sci., 1977, vol. 17, pp. 269-99.
D. Lai, R.J. Borg, M.J. Brabers, J.D. Mackenzie, and C.E. Birchenall: Corrosion, 1961, vol. 17, pp. 357-64.
A.K. Shukla, D. Gond, M. Bharadwaj, and D. Puri: J. Miner. Mater. Charact. Eng., 2011, vol. 10, pp. 1061-75.
S.G. Wang, M. Sun, H.B. Han, K. Long, and Z.D. Zhang: Corros. Sci., 2013, vol. 72, pp. 64-72.
G. Bamba, Y. Wouters, A. Galerie, F. Charlot, and A. Dellali, Acta Mater., 2006, vol. 54, pp. 3917-22.
A. Safikhani, M. Esmailian, M.R. Salmani and M. Aminfard: Int. J. Hydrogen Energy, 2014, vol. 39, pp. 11210-23.
X. Cheng, Z. Jiang, B.J. Monaghan, D. Wei, R.J. Longbottom, J. Zhao, and J. Jiang: Corros. Sci., 2016, vol. 108, pp. 11-22.
V. Lepingle, G. Louis, D. Allué, B. Lefebvre, and B. Vandenberghe: Corros. Sci., 2008, vol. 50, pp. 1011-19.
A.S. Khanna, P. Rodriguez, and J.B. Gnanamoorthy: Oxid. Met., 1986, vol. 26, pp. 171-200.
D.L.A. De Faria, S. Venâncio Silva, and M.T. De Oliveira: J. Raman Spectrosc., 1997, vol. 28, pp. 873-78.
K.F. McCarty and D.R. Boehme: J. Solid State Chem., 1989, vol. 79, pp. 19-27.
M.H.B. Ani, T. Kodama, M. Ueda, K. Kawamura, and T. Maruyama: Mater. Trans., 2009, vol. 50, pp. 2656-63.
A. Bowen and G. Leak: Metall. Trans., 1970, vol. 1, pp. 2767-73.
M.P. Shor: The Design of a functionally graded composite for service in high temperature lead and lead-bismuth cooled nuclear reactors, Massachusetts Institute of Technology, 2010.
J.H. Kim, D.I. Kim, J.H. Shim, and K.W. Yi: Corros. Sci., 2013, vol. 77, pp. 397-402.
A.P. Greeff, C.W. Louw, and H.C. Swar: Corros. Sci., 2000, vol. 42, pp. 1725-40.
B. Hua, Y. Kong, W. Zhang, J. Pu, B. Chi, and L. Jian: J. Power Sources, 2011, vol. 196, pp. 7627-38.
N.K. Othman, J. Zhang, and D.J. Young: Corros. Sci., 2010, vol. 52, pp. 2827-36.
G. Salje and M. Feller-Kniepmeier: J. Appl. Phys., 1977, vol. 48, pp. 1833-39.
D.G. Barnes, J.M. Calvert, K.A. Hay, and D.G. Lees: Philos. Mag., 1973, vol. 28, pp. 1303-18.
N. Birks, G.H. Meier, and F.S. Pettit: Introduction to the high-temperature oxidation of metals, 2nd ed., Cambridge University Press, New York, USA, 2006.
A. Brückman and J. Romanski: Corros. Sci., 1965, vol. 5 pp. 185-91.
E. Huttunen-Saarivirta, V.T. Kuokkala, and P. Pohjanne: Corros. Sci., 2014, vol. 87, pp. 344-65.
K.C. Chou: J. Am. Ceram. Soc., 2006, vol. 89, pp. 1568-76.
X.M. Hou, E.H. Wang, Y.X. Liu, and K.C. Chou: Metall. Mater. Trans. A, 2015, vol. 46, pp. 1621-27.
Acknowledgments
The authors express their appreciation to the National Science Foundation for Excellent Young Scholars of China (No. 51522402), the National Science Foundation of China (No. 51572019 and U1460201), the Special Fund of the National Excellent Doctoral Dissertation (No. 201437), the National Key Research and Development Program (2017YFB0304905), and the Central Universities of No. FRF-TP-15-006C1 for financial support. In addition, the authors thank Liwen Bianji, Edanz Group China (www.liwenbianji.cn/ac), for editing the English text of a draft of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Manuscript submitted December 4, 2017.
Rights and permissions
About this article
Cite this article
Wang, E., Cheng, J., Ma, J. et al. Effect of Temperature on the Initial Oxidation Behavior and Kinetics of 5Cr Ferritic Steel in Air. Metall Mater Trans A 49, 5169–5179 (2018). https://doi.org/10.1007/s11661-018-4781-2
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11661-018-4781-2