Abstract
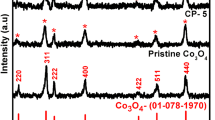
Dopant-free Co(OH)2 was synthesized from a precursor of 0.1 M CoCl2 on the single-sided conducting surface area of 1 cm2 of stainless steel strip (SSS) substrate. It was developed by using a low-cost two-electrode technique at 0.5 V, 28 °C, 50 s reaction time, and in 50 ml double distilled water. Further, electrochemical properties are measured by a three-electrode technique. The charge/discharge characteristic testing of Co(OH)2 at different current densities (1 A/g, 3 A/g, 5 A/g) were studied which results in shifting of peaks toward the positive direction. CV examination of Co(OH)2 material as electrode was carried out at different scan rates (10 mV/s, 20 mV/s, 30 mV/s) in 0.1 M potassium hydroxide solution ranging − 0.2 to 0.5 V. A pair of redox peaks was noticeably observed in CV trace, demonstrating the typical capacitive behavior of Co(OH)2 electrode. The specific capacitances (87.5 to 262.5 F/g), specific energy densities (28 to 84 Wh/kg), and specific power densities (0.08 to 0.24 W/kg) were measured from GCD. Additionally, morphological studies such as XRD, SEM, and EDS were investigated. The morphological results show Co(OH)2 is having crystalline structure, average crystallite size of 37.88 nm, particle size of 180 nm, and elemental analysis confirms the presence of the elements. Further, it can be concluded that the Co(OH)2 has good electrochemical performance which is suitable for supercapacitor electrode material. This cobalt hydroxide electrode is a favourable candidate for electrochemical energy-storage devices.
Graphical abstract









Similar content being viewed by others
Data availability
No applicable.
Abbreviations
- XRD :
-
X-ray diffraction
- FESEM :
-
field emission scanning electron microscopy
- CV :
-
cyclic voltammetry
- EIS :
-
electrical impedance spectroscopy
- EDS :
-
energy dispersive X-ray spectroscopy
- GCD :
-
galvanostatic charge discharge
- SSS :
-
stainless steel strip
- CoCl 2 :
-
cobalt chloride
- Co(OH) 2 :
-
cobalt hydroxide
References
Zhao T, Jiang H, Ma J (2011) Surfactant-assisted electrochemical deposition of α-cobalt hydroxide for supercapacitors. J Power Sources 196(2):860–864. https://doi.org/10.1016/j.jpowsour.2010.06.042
Kelpšaitė I, Baltrušaitis J, Valatka E (2011) Electrochemical deposition of porous cobalt oxide films on AISI 304 type steel. Mater Sci 17(3):236–243. https://doi.org/10.5755/j01.ms.17.3.586
Jebakumar Immanuel Edison TN, Atchudan R, Perumal S, Bothi Raja P, Perumal V, Lee YR (2022) Electrodeposition of cobalt oxide nanoparticles on stainless steel for electrocatalytic water oxidation. Top Catal:1–11. https://doi.org/10.1007/s11244-022-01754-8
Jayashree RS, Kamath PV (1999) Electrochemical synthesis of α-cobalt hydroxide. J Mater Chem 9(4):961–963. https://doi.org/10.1039/A807000H
Deng T, Zhang W, Arcelus O, Kim JG, Carrasco J, Yoo SJ et al (2017) Atomic-level energy storage mechanism of cobalt hydroxide electrode for pseudocapacitors. Nat Commun 8(1):15194. https://doi.org/10.1038/ncomms15194
Jagadale A, Kumbhar V, Dhawale D, Lokhande C (2013) Performance evaluation of symmetric supercapacitor based on cobalt hydroxide [Co(OH)2] thin film electrodes. Electrochim Acta 98:32–38. https://doi.org/10.1016/j.electacta.2013.02.094
Aouini S, Bardaoui A, do Rego AM, Ferraria AM, Santos DM, Chtourou R (2023) Synthesis and characterization of CoMn2O4 spinel onto flexible stainless-steel mesh for supercapacitor application. Solid State Sci 143:107283. https://doi.org/10.1016/j.solidstatesciences.2023.107
Aouini S, Bardaoui A, Ferraria AM, Santos DM, Chtourou R (2022) ZnMn2O4 nanopyramids fabrication by hydrothermal route: effect of reaction time on the structural, morphological, and electrochemical properties. Energies 15(24). https://doi.org/10.3390/en15249352
Aouini S, Bardaoui A, Santos DM, Chtourou R (2022) Hydrothermal synthesis of CuMn2O4 spinel-coated stainless steel mesh as a supercapacitor electrode. J Mater Sci Mater Electron 33(16):12726–12733. https://doi.org/10.1007/s10854-022-08219-4
Gupta V, Gupta S, Miura N (2008) Al-substituted α-cobalt hydroxide synthesized by potentiostatic deposition method as an electrode material for redox-supercapacitors. J Power Sources 177(2):685–689. https://doi.org/10.1016/j.jpowsour.2007.10.091
Sebastian M, Nethravathi C, Rajamathi M (2013) Interstratified hybrids of α-hydroxides of nickel and cobalt as supercapacitor electrode materials. Mater Res Bull 48(7):2715–2719. https://doi.org/10.1016/j.materresbull.2013.03.029
Zhou WJ, Zhang J, Xue T, Zhao DD, Li HL (2008) Electrodeposition of ordered mesoporous cobalt hydroxide film from lyotropic liquid crystal media for electrochemical capacitors. J Mater Chem 18(8):905–910. https://doi.org/10.1039/B715070A
Choi D, Blomgren GE, Kumta PN (2006) Fast and reversible surface redox reaction in nanocrystalline vanadium nitride supercapacitors. Adv Mater 18(9):1178–1182. https://doi.org/10.1002/adma.200502471
Casella IG, Gatta M (2002) Study of the electrochemical deposition and properties of cobalt oxide species in citrate alkaline solutions. J Electroanal Chem 534(1):31–38. https://doi.org/10.1016/S0022-0728(02)01100-2
Wang XF, You Z, Ruan DB (2006) A hybrid metal oxide supercapacitor in aqueous KOH electrolyte. Chin J Chem 24(9):1126–1132. https://doi.org/10.1002/cjoc.200690212
Hu ZA, Xie YL, Wang YX, Xie LJ, Fu GR, Jin XQ et al (2009) Synthesis of α-cobalt hydroxides with different intercalated anions and effects of intercalated anions on their morphology, basal plane spacing, and capacitive property. J Phys Chem C 113(28):12502–12508. https://doi.org/10.1021/jp8106809
Xu ZP, Zeng HC (1999) Interconversion of brucite-like and hydrotalcite-like phases in cobalt hydroxide compounds. Chem Mater 11(1):67–74. https://doi.org/10.1021/cm980420b
Pell WG, Conway BE (2001) Voltammetry at a de Levie brush electrode as a model for electrochemical supercapacitor behaviour. J Electroanal Chem 500(1-2):121–133. https://doi.org/10.1016/S0022-0728(00)00423-X
Lokhande PE, Kulkarni S, Chakrabarti S, Pathan HM, Sindhu M, Kumar D et al (2022) The progress and roadmap of metal–organic frameworks for high-performance supercapacitors. Coord Chem Rev 473:214771. https://doi.org/10.1016/j.ccr.2022.214771
Liang YY, Li HL, Zhang XG (2008) A novel asymmetric capacitor based on Co (OH) 2/USY composite and activated carbon electrodes. Mater Sci Eng A 473(1-2):317–322. https://doi.org/10.1016/j.msea.2007.03.087
Jagadale AD, Kumbhar VS, Bulakhe RN, Lokhande CD (2014) Influence of electrodeposition modes on the supercapacitive performance of Co3O4 electrodes. Energy 64:234–241. https://doi.org/10.1016/j.energy.2013.10.016
Herrera-Zamora DM, Lizama-Tzec FI, Santos-González I, Rodríguez-Carvajal RA, García-Valladares O, Arés-Muzio O, Oskam G (2020) Electrodeposited black cobalt selective coatings for application in solar thermal collectors: fabrication, characterization, and stability. Sol Energy 207:1132–1145. https://doi.org/10.1016/j.solener.2020.07.042
Sun S, Xu ZJ (2015) Composition dependence of methanol oxidation activity in nickel–cobalt hydroxides and oxides: an optimization toward highly active electrodes. Electrochim Acta 165:56–66. https://doi.org/10.1016/j.electacta.2015.03.008
Khavale S, Lokhande B (2018) Symmetric supercapacitor device prepared using Koh as liquid electrolyte and potentiodynamically electrodeposited Mn incorporated Co3O4. Int J Ind Electr Electron Eng 6:1 http://ijieee.org.in/volume.php?volume_id=433
Mhetre MT, Pathan HM, Thakur AV, Lokh BJ (2022) Preparation of magnesium oxide (MgO) thin films by spray pyrolysis and its capacitive characterizations. ES Energy Environ 18:41–46. https://doi.org/10.30919/esee8c785
Ghadge TS, Jadhav AL, Uplane YM, Thakur AV, Kamble SV, Lokhande BJ (2021) Controlled synthesis, structural, morphological and electrochemical study of Cu (OH) 2@ Cu flexible thin film electrodes prepared via aqueous–non-aqueous routes. J Mater Sci Mater Electron 32:9018–9031. https://doi.org/10.1007/s10854-021-05572-8
Thakur AV, Lokhande BJ (2018) Source molarity affected surface morphological and electrochemical transitions in binder-free FeO (OH) flexible electrodes and fabrication of symmetric supercapacitive device. Chem Pap 72(6):1407–1415. https://doi.org/10.1007/s11696-018-0383-0
Thakur AV, Lokhande BJ (2018) Morphological modification for optimum electrochemical performance of highly pristine polypyrrole flexible electrodes, via SILAR immersion time and fabrication of solid state symmetric device. Port Electrochim Acta 36(6):377–392. https://doi.org/10.4152/pea.201806377
Thakur AV, Lokhande BJ (2018) Synthesis and electrolytic cation-dependent cyclic voltammetric study of SILAR deposited PPy-Cr 2 O 3 in equimolar aqueous solutions of H 2 SO 4, Na 2 SO 4, and K 2 SO 4. Mater Renew Sustain Energy 7:1–8. https://doi.org/10.1007/s40243-018-0125-9
Thakur AV, Pathan HM, Lokh BJ (2022) Optimization of symmetric supercapacitors by controlled in-situ growth of RuO2 within PPy matrix tailored by RuCl3% during SILAR. ES Energy Environ 18:75–89. https://doi.org/10.30919/esee8c751
Thakur AV, Lokhande BJ (2021) Study of RuO 2 incorporated PPy hybrid flexible electrodes prepared by soaking and drying technique: a novel approach. Appl Phys A 127:1–6. https://doi.org/10.1007/s00339-021-05028-0
Kambale, S. V., Jadhav, A. L., Kore, R. M., Thakur, A. V., & Lokhande, B. J. (2019). Cyclic voltammetric study of CuO thin film electrodes prepared by automatic spray pyrolysis. In Macromolecular Symposia (Vol. 387, No. 1, p. 1800213). https://doi.org/10.1002/masy.201800213
Thakur AV, Lokhande BJ (2019) Effect of the molar concentration of pyrrole monomer on the rate of polymerization, growth and hence the electrochemical behavior of highly pristine PPy flexible electrodes. Heliyon 5(11):e02909. https://doi.org/10.1016/j.heliyon.2019.e02909
Thakur AV, Lokhande BJ (2017) Effect of dip time on the electrochemical behavior of PPy-Cu (OH) 2 hybrid electrodes synthesized using pyrrole and CuSO4. e-Polym 17(2):167–173. https://doi.org/10.1515/epoly-2016-0160
Thakur AV, Lokhande BJ (2017) Dip time-dependent SILAR synthesis and electrochemical study of highly flexible PPy-Cu (OH) 2 hybrid electrodes for supercapacitors. J Solid State Electrochem 21:2577–2584. https://doi.org/10.1007/s10008-016-3502-2
Thakur AV, Lokhande BJ (2017) Electrolytic anion affected charge storage mechanisms of Fe 3 O 4 flexible thin film electrode in KCl and KOH: a comparative study by cyclic voltammetry and galvanostatic charge–di. J Mater Sci Mater Electron 28:11755–11761. https://doi.org/10.1007/s10854-017-6980-9
Thakur AV, Lokhande BJ (2019) Effect of precursor bath temperature on the morphology and electrochemical performance of SILAR-synthesized PPy: FeOOH hybrid flexible electrodes. Chem Pap 73:833–841. https://doi.org/10.1007/s11696-018-0644-y
Patil UM, Nam MS, Sohn JS, Kulkarni SB, Shin R, Kang S et al (2014) Controlled electrochemical growth of Co (OH) 2 flakes on 3D multilayered graphene foam for high performance supercapacitors. J Mater Chem A 2(44):19075–19083. https://doi.org/10.1039/C4TA03953J
Vidhya MS, Ravi G, Yuvakkumar R, Velauthapillai D, Thambidurai M, Dang C, Saravanakumar B (2020) Nickel–cobalt hydroxide: a positive electrode for supercapacitor applications. RSC Adv 10(33):19410–19418. https://doi.org/10.1039/D0RA01890B
Acknowledgements
We would like to thank Dr. Habib M. Pathan (Associate Professor, Advanced Physical Laboratory, Department of Physics, Savitribai Phule Pune University, Pune) for providing us with lab facilities to perform experiment work at Advanced Physics Laboratory. We are also thankful to Dr. Aqueel Ahmad Shah (Principal, Maulana Mukhtar Ahmad Nadvi Technical Campus) for motivating us for research work.
Author information
Authors and Affiliations
Contributions
SN has done experiment work to prepare the cobalt hydroxide thin films. AS has completed the characterizations. AR has written the literature and introduction section of the manuscript. DH has assisted in the results and discussions section. TA has improved the writing of the manuscript. AP has contributed to the manuscript as a supervisor. All authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Naeem, S., Shaikh, A., Rasool, A. et al. Enhancing supercapacitor performance through electrodeposition of cobalt hydroxide thin film: structural analysis, morphological characterization, and investigation of electrochemical properties. Ionics 30, 399–405 (2024). https://doi.org/10.1007/s11581-023-05293-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-023-05293-4