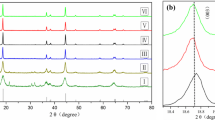
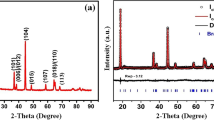
Abstract
The research of high-performance cathode materials for rechargeable lithium-ion batteries (LIBs) is highly desirable. The ternary layered oxide LiNi1/3Co1/3Mn1/3O2 (LNCM) is a promising cathode material for LIBs due to its high discharge voltage, large specific capacity, good thermostability, and low cost. However, the LNCM cathode still has certain limitations, including cationic mixing and low electronic conductivity. These drawbacks ultimately result in poor cycling stability, rapid voltage degradation, and capacity loss during high-rate cycling. To address these issues, we have established a feasible sol-gel method combined with calcination to prepare LNCM, which can significantly improve the electrochemical activity of the LNCM cathode. The developed LNCM−850/10 cathode displays an initial specific discharge capacity of 215.3 mAh g−1 at a current rate of 0.2 C and retains a high reversible capacity of 93.9 mAh g−1 after 200 cycles. In addition, the LNCM−850/10 cathode also exhibits excellent high-rate charge-discharge capability and high-rate cycling performance. These remarkable results are probably due to the low Li+/Ni2+ cation mixing degree, good particle morphology, and uniform particle size distribution of LNCM−850/10, which effectively improves the electronic conductivity and lowers the charge transfer resistance, while reducing the Li+ diffusion distance and accelerating the insertion/extraction of Li+. Our study demonstrates that careful control of the calcination temperature of sol-gel-synthesized LNCM precursors can promote the development of LNCM cathodes suitable for advanced LIBs.






Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding authors upon reasonable request.
References
Ome AM (2008) Energy, environment and sustainable development. Renew Sust Energ Rev 12:2265–2300. https://doi.org/10.1016/j.rser.2007.05.001
Talaat M, Farahat MA, Elkholy MH (2019) Renewable power integration: experimental and simulation study to investigate the ability of integrating wave, solar and wind energies. Energy 170:668–682. https://doi.org/10.1016/j.energy.2018.12.171
Zhou T, Xie LL, Niu Y, Xiao HR, Li YJ, Han Q, Qiu XJ, Yang XL, Wu XY, Zhu LM, Pang H, Cao XY (2023) New insights on (V10O28)6--based electrode materials for energy storage: a brief review. Rare Met 42:1431–1445. https://doi.org/10.1007/s12598-022-02207-7
Harper G, Sommerville R, Kendrick E, Driscoll L, Slater P, Stolkin R, Walton A, Christensen P, Herdrich O, Lambert S, Abbott A, Ryder KS, Gaines L, Anderson P (2019) Recycling lithium-ion batteries from electric vehicles. Nature 575:75–86. https://doi.org/10.1038/s41586-019-1682-5
Wu F, Maier J, Yu Y (2020) Guidelines and trends for next-generation rechargeable lithium and lithium-ion batteries. Chem Soc Rev 49:1569–1614. https://doi.org/10.1039/c7cs00863e
Liu H, Liu X, Li W, Guo X, Wang Y, Wang G, Zhao D (2017) Porous carbon composites for next generation rechargeable lithium batteries. Adv Energy Mater 7:1700283. https://doi.org/10.1002/aenm.201700283
Li M, Lu J, Chen Z, Amine K (2018) 30 years of lithium-ion batteries. Adv Mater 30:1800561. https://doi.org/10.1002/adma.201800561
Zhu LM, Ding GC, Han Q, Miao YX, Li X, Yang XL, Chen L, Wang GK, Xie LL, Cao XY (2022) Enhancing electrochemical performances of small quinone toward lithium and sodium energy storage. Rare Met 41:425–437. https://doi.org/10.1007/s12598-021-01813-1
Wu ZP, Wang YL, Liu XB, Lv C, Li YS, Wei D, Liu ZF (2019) Carbon-nanomaterial-based flexible batteries for wearable electronics. Adv Mater 31:1800716. https://doi.org/10.1002/adma.201800716
Zhang W, Wang L, Ding G, Yang Y, Yang G, Xu J, Xu N, Xie L, Han Q, Zhu L, Cao X, Ma J (2023) Bimetallic CoNiSe2/C nanosphere anodes derived from Ni-Co-metal-organic framework precursor towards higher lithium storage capacity. Chin Chem Lett 34:107328. https://doi.org/10.1016/j.cclet.2022.03.051
Zhu L, Ding G, Xie L, Cao X, Liu J, Lei X, Ma J (2019) Conjugated carbonyl compounds as high-performance cathode materials for rechargeable batteries. Chem Mater 31:8582–8612. https://doi.org/10.1021/acs.chemmater.9b03109
Chen Y, Kang Y, Zhao Y, Wang L, Liu J, Li Y, Liang Z, He X, Li X, Tavajohi N, Li B (2021) A review of lithium-ion battery safety concerns: the issues, strategies, and testing standards. J Energy Chem 59:83–99. https://doi.org/10.1016/j.jechem.2020.10.017
Lee W, Muhammad S, Sergey C, Lee H, Yoon J, Kang YM, Yoon WS (2020) Advances in the cathode materials for lithium rechargeable batteries. Angew Chem Int Ed 59:2578–2605. https://doi.org/10.1002/anie.201902359
Biasi LD, Schwarz B, Brezesinski T, Hartmann P, Janek J, Ehrenberg H (2019) Chemical, structural, and electronic aspects of formation and degradation behavior on different length scales of Ni-rich NCM and Li-rich HE-NCM cathode materials in Li-ion batteries. Adv Mater 31:1900985. https://doi.org/10.1002/adma.201900985
Yin S, Deng W, Chen J, Gao X, Zou G, Hou H, Ji X (2021) Fundamental and solutions of microcrack in Ni-rich layered oxide cathode materials of lithium-ion batteries. Nano Energy 83:105854. https://doi.org/10.1016/j.nanoen.2021.105854
Wang L, Wang Z, Xie L, Zhu L, Cao X (2019) ZIF-67-derived N-doped Co/C nanocubes as high-performance anode materials for lithium-ion batteries. ACS Appl Mater Interfaces 18:16619–16628. https://doi.org/10.1021/acsami.9b03365
Tian C, Lin F, Doeff MM (2018) Electrochemical characteristics of layered transition metal oxide cathode materials for lithium ion batteries: surface, bulk behavior, and thermal properties. Acc Chem Res 51:89–96. https://doi.org/10.1021/acs.accounts.7b00520
Sun YK, Chen Z, Noh HJ, Lee DJ, Jung HG, Ren Y, Wang S, Yoon CS, Myung ST, Amine K (2012) Nanostructured high-energy cathode materials for advanced lithium batteries. Nat Mater 11:942–947. https://doi.org/10.1038/NMAT3435
He P, Yu H, Li D, Zhou H (2012) Layered lithium transition metal oxide cathodes towards high energy lithium-ion batteries. J Mater Chem A 22:3680–3695. https://doi.org/10.1039/c2jm14305d
Browne MP, Sofer Z, Pumera M (2019) Layered and two dimensional metal oxides for electrochemical energy conversion. Energy Environ Sci 12:41–58. https://doi.org/10.1039/c8ee02495b
Tan S, Shadike Z, Li J, Wang X, Yang Y, Lin R, Cresce A, Hu J, Hunt A, Waluyo I (2022) Additive engineering for robust interphases to stabilize high-Ni layered structures at ultra-high voltage of 4.8 V. Nat Energy 7:484–494. https://doi.org/10.1038/s41560-022-01020-x
Liu W, Oh P, Liu X, Lee MJ, Cho W, Chae S, Kim Y, Cho J (2015) Nickel-rich layered lithium transition-metal oxide for high-energy lithium-ion batteries. Angew Chem Int Ed 54:4440–4457. https://doi.org/10.1002/anie.201409262
Koksbang R, Barker J, Shi H (1996) Cathode materials for lithium rocking chair batteries. Solid State Ion 84:1–21. https://doi.org/10.1016/S0167-2738(96)83001-3
Lyu Y, Wu X, Wang K, Feng Z, Cheng T, Liu Y, Wang M, Chen R, Xu L, Zhou J, Lu Y, Guo B (2021) An overview on the advances of LiCoO2 cathodes for lithium-ion batteries. Adv Energy Mater 11:2000982. https://doi.org/10.1002/aenm.202000982
Okubo M, Hosono E, Kim J, Enomoto M, Kojima N, Kudo T, Zhou H, Honma I (2007) Nanosize effect on high-rate Li-ion intercalation in LiCoO2 electrode. J Am Chem Soc 129:7444–7452. https://doi.org/10.1021/ja0681927
Zhang J, Li Q, Ouyang C, Yu X, Ge M, Huang X, Hu E, Ma C, Li S, Xiao R, Yang W, Chu Y, Liu Y, Yu H, Yang X, Huang X, Chen L, Li H (2019) Trace doping of multiple elements enables stable battery cycling of LiCoO2 at 4.6 V. Nat Energy 4:594–603. https://doi.org/10.1038/s41560-019-0409-z
Zhang J, Wang PF, Bai P, Wan H, Liu S, Hou S, Pu X, Xia J, Zhang W, Wang Z, Nan B, Zhang X, Xu J, Wang C (2022) Interfacial design for a 4.6 V high-voltage single-crystalline LiCoO2 cathode. Adv Mater 34:2108353. https://doi.org/10.1002/adma.202108353
Wang L, Li L, Zhang X (2018) Compound-hierarchical-sphere LiNi0.5Co0.2Mn0.3O2: synthesis, structure, and electrochemical characterization. ACS Appl Mater Interfaces 10:32120–32127. https://doi.org/10.1021/acsami.8b09985
Zheng JM, Yan PF, Estevez L, Wang CM, Zhang JG (2018) Effect of calcination temperature on the electrochemical properties of nickel-rich LiNi0.76Mn0.14Co0.10O2 cathodes for lithium-ion batteries. Nano Energy 49:538–548. https://doi.org/10.1016/j.nanoen.2018.04.077
Li W, Asl HY, Xie Q, Manthiram A (2019) Collapse of LiNi1-x-yCoxMnyO2 lattice at deep charge irrespective of nickel content in lithium-ion batteries. J Am Chem Soc 141:5097–5101. https://doi.org/10.1021/jacs.8b13798
Chakraborty A, Kunnikuruvan S, Kumar S, Markovsky B, Aurbach D, Dixit M, Majo DT (2020) Review of computational studies on LiNi1-x-yCoxMnyO2 and LiNi1-x-yCoxAlyO2. Chem Mater 32:915–952. https://doi.org/10.1021/acs.chemmater.9b04066
Zuo TT, Rueß R, Pan R, Walther F, Rohnke M, Hori S, Kanno R, Schröder D, Janek J (2021) A mechanistic investigation of the Li10GeP2S12|LiNi1-x-yCoxMnyO2 interface stability in all-solid-state lithium batteries. Nat Commun 12:6669. https://doi.org/10.1038/s41467-021-26895-4
Trevisanello E, Ruess R, Conforto G, Richter FH, Janek J (2021) Polycrystalline and single crystalline NCM cathode materials-quantifying particle cracking, active surface area, and lithium diffusion. Adv Energy Mater 11:2003400. https://doi.org/10.1002/aenm.202003400
Hua WB, Guo XD, Zheng Z, Wang YJ, Zhong BH, Fang B, Wang JZ, Chou SL, Liu H (2015) Uncovering a facile large-scale synthesis of LiNi1/3Co1/3Mn1/3O2 nanoflowers for high power lithium-ion batteries. J Power Sources 275:200–206. https://doi.org/10.1016/j.jpowsour.2014.09.178
Lee GH, Wu J, Kim D, Cho K, Cho M, Yang W, Kang YM (2020) Reversible anionic redox activities in conventional LiNi1/3Co1/3Mn1/3O2 cathodes. Angew Chem Int Ed 59:8681–8688. https://doi.org/10.1002/anie.202001349
Cao X, Zhao Y, Zhu L, Xie L, Cao X, Xiong S, Wang C (2016) Synthesis and characterization of LiNi1/3Co1/3Mn1/3O2 as cathode materials for Li-ion batteries via an efficacious sol-gel method. Int J Electrochem Sci 11:5267–5278. https://doi.org/10.20964/2016.06.93
Chen Y, Zhao W, Zhang Q, Yang G, Zheng J, Tang W, Xu Q, Lai C, Yang J, Peng C (2020) Armoring LiNi1/3Co1/3Mn1/3O2 cathode with reliable fluorinated organic-inorganic hybrid interphase layer toward durable high rate battery. Adv Funct Mater 30:2000396. https://doi.org/10.1002/adfm.202000396
Liang C, Jiang L, Ye S, Wang Z, Wei Z, Wang Q, Sun J (2021) Precise in-situ and ex-situ study on thermal behavior of LiNi1/3Co1/3Mn1/3O2/graphite coin cell: from part to the whole cell. J Energy Chem 54:332–341. https://doi.org/10.1016/j.jechem.2020.06.008
Zhu L, Bao C, Xie L, Yang X, Cao X (2020) Review of synthesis and structural optimization of LiNi1/3Co1/3Mn1/3O2 cathode materials for lithium-ion batteries applications. J Alloys Compd 831:154864. https://doi.org/10.1016/j.jallcom.2020.154864
Luo B, Jiang B, Peng P, Huang J, Chen J, Li M, Chu L, Li Y (2019) Improving the electrochemical performance of LiNi1/3Co1/3Mn1/3O2 cathode material via tungsten modification. Electrochim Acta 297:398–405. https://doi.org/10.1016/j.electacta.2018.11.202
Li G, Huang Z, Zuo Z, Zhang Z, Zhou H (2015) Understanding the trace Ti surface doping on promoting the low temperature performance of LiNi1/3Co1/3Mn1/3O2 cathode. J Power Sources 281:69–76. https://doi.org/10.1016/j.jpowsour.2015.01.173
Lv C, Yang J, Peng Y, Duan X, Ma J, Li Q, Wang T (2019) 1D Nb-doped LiNi1/3Co1/3Mn1/3O2 nanostructures as excellent cathodes for Li-ion battery. Electrochim Acta 297:258–266. https://doi.org/10.1016/j.electacta.2018.11.172
Cong L, Zhao Q, Wang Z, Zhang Y, Wu X, Zhang J, Wang R, Xie H, Sun L (2016) (PO4)3- polyanions doped LiNi1/3Co1/3Mn1/3O2: an ultrafast-rate, long-life and high-voltage cathode material for Li-ion rechargeable batteries. Electrochim Acta 201:8–19. https://doi.org/10.1016/j.electacta.2016.03.088
Lei Y, Li Y, Jiang H, Lai C (2019) Preparing enhanced electrochemical performances Fe2O3-coated LiNi1/3Co1/3Mn1/3O2 cathode materials by thermal decomposition of iron citrate. J Mater Sci 54:4202–4211. https://doi.org/10.1007/s10853-018-3126-2
Wang X, Jiang Q, Zhang Y, Yuan N, Tang J (2020) High efficient and environment friendly plasma-enhanced synthesis of Al2O3-coated LiNi1/3Co1/3Mn1/3O2 with excellent electrochemical performance. Front Chem 8:72. https://doi.org/10.3389/fchem.2020.00072
Zhu L, Xie L, Bao C, Yan X, Cao X (2020) LiNi1/3Co1/3Mn1/3O2/polypyrrole composites as cathode materials for high-performance lithium-ion batteries. Int J Energy Res 44:298e308. https://doi.org/10.1002/er.4916
Cheng C, Chen F, Yi H, Lai G (2018) Enhanced electrochemical and safe performances of LiNi1/3Co1/3Mn1/3O2 by nano-CeO2 coating via a novel hydrolysis precipitate reaction route. J Alloys Compd 753:155–161. https://doi.org/10.1016/j.jallcom.2018.04.229
Jiang Q, Lang P, Li J, Tang J (2018) Improving the elevated-temperature behaviors of LiNi1/3Co1/3Mn1/3O2 by surface modification with Nano-La2O3. J Alloys Compd 742:549–554. https://doi.org/10.1016/j.jallcom.2018.01.354
Cui Y, Yang C, Zhuang Z, Wang M, Zhuang Q (2018) Synthesis and electrochemical performance of spheroid LiNi1/3Co1/3Mn1/3O2 in the electrolyte modified by ethylene sulfate and methylene methanedisulfonate. J Inorg Organomet P 28:731–737. https://doi.org/10.1007/s10904-017-0722-6
Fang Y, Huang Y, Tong W, Cai Y, Wang X, Guo Y, Jia D, Zong J (2018) Synthesis of hollow peanut-like hierarchical mesoporous LiNi1/3Co1/3Mn1/3O2 cathode materials with exceptional cycle performance for lithium-ion batteries by a simple self-template solid-state method. J Alloys Compd 743:707–715. https://doi.org/10.1016/j.jallcom.2018.01.257
Li J, Yao R, Cao C (2014) LiNi1/3Co1/3Mn1/3O2 nanoplates with {010} active planes exposing prepared in polyol medium as a high-performance cathode for Li-ion battery. ACS Appl Mater Interfaces 6:5075–5082. https://doi.org/10.1021/am500215b
Zhang Y, Zhang W, Shen S, Yan X, Wu R, Wu A, Lastoskie C, Zhang J (2017) Sacrificial template strategy toward a hollow LiNi1/3Co1/3Mn1/3O2 nanosphere cathode for advanced lithium-ion batteries. ACS Omega 2:7593–7599. https://doi.org/10.1021/acsomega.7b00764
Zhou H, Cheng H, Zhao H, Zhao K, Zhao Y, Zhang J, Xu Q, Lu X (2019) Superior stability and dynamic performance of single crystal LiNi1/3Co1/3Mn1/3O2 nanorods from beta-MnO2 template for lithium-ion batteries. J Electrochem Soc 166:A59–A67. https://doi.org/10.1149/2.0281902jes
Zhu L, Yang G, Liu J, Bao C, Xie L, Cao X (2019) Ethylene glycol-assisted sol-gel method for preparing LiNi1/3Co1/3Mn1/3O2 as cathode material for lithium-ion batteries with excellent electrochemical performance. ChemistrySelect 4:11475–11482. https://doi.org/10.1002/slct.201903231
Zheng H, Chen X, Yang Y, Li L, Li G, Guo Z, Feng C (2017) Self-assembled LiNi1/3Co1/3Mn1/3O2 nanosheet cathode with high electrochemical performance. ACS Appl Mater Interfaces 9:39560–39568. https://doi.org/10.1021/acsami.7b10264
Jiang X, Sha Y, Cai R, Shao Z (2015) The solid-state chelation synthesis of LiNi1/3Co1/3Mn1/3O2 as a cathode material for lithium-ion batteries. J Mater Chem A 3:10536–10544. https://doi.org/10.1039/C5TA01236H
Zhang CF, Yang P, Dai X, Xiong X, Zhan J, Zhang YL (2009) Synthesis of LiNi1/3Co1/3Mn1/3O2 cathode material via oxalate precursor. T Nonferr Metal Soc 19:635–641. https://doi.org/10.1016/S1003-6326(08)60325-8
Fujii Y, Miura H, Suzuki N, Shoji T, Nakayama N (2007) Structural and electrochemical properties of LiNi1/3Co1/3Mn1/3O2: calcination temperature dependence. J Power Sources 171:894–903. https://doi.org/10.1016/j.jpowsour.2007.06.017
Zeng J, Hai C, Ren X, Li X, Shen Y, Dong O, Zhang L, Sun Y, Ma L, Zhang X, Dong S, Zhou Y (2018) Facile triethanolamine-assisted combustion synthesized layered LiNi1/3Co1/3Mn1/3O2 cathode materials with enhanced electrochemical performance for lithium-ion batteries. J Alloys Compd 735:1977–1985. https://doi.org/10.1016/j.jallcom.2017.11.321
Fan G, Liu Z, Feng L, Feng L, Yang W, Liu B (2018) Understanding capacity fade of LiNi1/3Co1/3Mn1/3O2 from microstructure in full lithium ion battery. J Electrochem Soc 165:A1943–A1949. https://doi.org/10.1149/2.1501809jes
Xu F, Yan H, Chen J, Zhang Z, Fan C (2018) Improving electrochemical properties of LiNi1/3Co1/3Mn1/3O2 by enhancing thermal decomposition of carbonates to synthesize ultrafine powders. J Electroanal Chem 820:118–122. https://doi.org/10.1016/j.jelechem.2018.04.069
Li Y, Hou X, Zhou Y, Han W, Liang C, Wu X, Wang S, Ru Q (2018) Electrochemical performance of structure-dependent LiNi1/3Co1/3Mn1/3O2 in aqueous rechargeable lithium-ion batteries. Energy Technol 6:391–396. https://doi.org/10.1002/ente.201700528
Li F, Kong L, Sun Y, Jin Y, Hou P (2018) Micron-sized monocrystalline LiNi1/3Co1/3Mn1/3O2 as high-volumetric-energy-density cathode for lithium-ion batteries. J Mater Chem A 6:12344–12352. https://doi.org/10.1039/c8ta03363c
Hou Q, Cao G, Wang P, Zhao D, Cui X, Li S, Li C (2018) Carbon coating nanostructured-LiNi1/3Co1/3Mn1/3O2 cathode material synthesized by chemical vapor deposition method for high performance lithium-ion batteries. J Alloys Compd 747:796–802. https://doi.org/10.1016/j.jallcom.2018.03.115
Wang H, Wei Y, Wang J, Long D (2018) Polymer-chelation synthesis of compositionally homogeneous LiNi1/3Co1/3Mn1/3O2 crystals for lithium-ion cathode. Electrochim Acta 269:724–732. https://doi.org/10.1016/j.electacta.2018.03.029
He L, Sun S, Yu J (2018) Performance of LiNi1/3Co1/3Mn1/3O2 prepared from spent lithium-ion batteries by a carbonate co-precipitation method. Ceram Int 44:351–357. https://doi.org/10.1016/j.ceramint.2017.09.180
Funding
This work was supported by the Natural Science Foundation of Henan, China (No. 222300420138), for the project entitled “Design, synthesis, and oxygen production performance of cobalt-titanium encapsulated polyoxotungstates with photocatalytic function orientation” and by the Ph.D. Programs Foundation of Henan University of Technology (No. 2021BS0027) for the project entitled “Polyoxometalate-based functional materials and their applications in the field of energy storage.”
Author information
Authors and Affiliations
Contributions
Qing Han: conceptualization, software, writing—original draft, funding acquisition. Chenguang Bao: methodology, investigation. Yongmei Xiao: data curation, validation. Xuejing Qiu: data curation, validation. Xinli Yang: resources, methodology, writing—review and editing, supervision.
Corresponding authors
Ethics declarations
Ethical approval
This paper is not applicable for both human and/or animal studies. This declaration is not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Han, Q., Bao, C., Xiao, Y. et al. Boosting the electrochemical performances of LiNi1/3Co1/3Mn1/3O2 cathodes via optimizing calcination temperature for lithium-ion batteries. Ionics 29, 4483–4493 (2023). https://doi.org/10.1007/s11581-023-05173-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-023-05173-x