Abstract
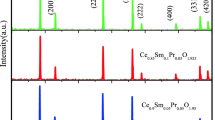
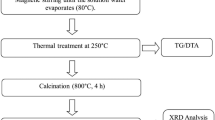
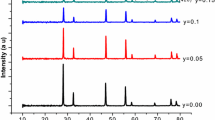
The different compositions of La3+ and In3+ co-doped ceria have been prepared by a citric acid-nitrate sol-gel method. All the compositions with general formula Ce0.85La0.15-xInxO1.925(CLI) have the same concentration of total oxygen vacancies. The results of the lattice parameter calculated by the hard sphere model are consistent with those of experimental data. All the samples sintered at 1350 °C have the relative density more than 96%. The relative density and grain size of all the sintered pellets increase with increasing In3+ concentration. Electrochemical analysis indicates that grain conductivity of Ce0.85La0.15-xInxO1.925 (x = 0.01~0.03) is pretty much the same with increasing content of In, while the grain boundary conductivity decreases with increasing content of In. Moreover, La3+ and In3+ co-doped exhibits higher ionic conductivity and lower activation energy as compared with La singly doped ceria. The maximum power density of based on the CLI1 cells co-fired at 1350 °C can reach 635 mW cm−2 at 700 °C.









Similar content being viewed by others
References
Wachsman ED, Marlowe CA, Lee KT (2012) Role of solid oxide fuel cells in a balanced energy strategy. Energy & Environ Sci 5:5498–5509
Steele BCH, Heinzel A (2001) Review article materials for fuel-cell technologies. Nature 414:345–352
Mahato N, Banerjee A, Gupta A, Omar S, Balani K (2015) Progress in material selection for solid oxide fuel cell technology: a review. Prog Mater Sci 72:141–337
Atkinson DJLA, Brandon NP, Skinner SJ (2008) Intermediate solid oxide fuel cells. Chem Soc Rev 37:1568–1578
Ding D, Li X, Lai SY, Gerdes K, Liu M (2014) Enhancing SOFC cathode performance by surface modification through infiltration. Energ & Environ Sci 7:552–575
Wachsman ED, Lee KT (2011) Lowering the temperature of solid oxide fuel cells. Science 334:935–939
PezColl D, Nunez P, Frade JR (2013) Effect of samarium content on onset of minor p-type conductivity in ceria-based electrolytes. J Power Sources 227:145–152
Wang Z, Shimizu S, Yamazaki Y (2008) Interconnection and sealing using silver metal for honeycomb SOFCs. J Fuel Cell Sci Tech 5:031211–031214
Peng R, Xia C, Liu X, Peng D, Meng G (2002) Intermediate-temperature SOFCs with thin Ce0.8Y0.2O1.9 films prepared byscreen-printing. Solid State Ionics 561:152–153
Oh EO, Whang CM, Lee YR, Park SY, Prasad DH, Yoon KJ, Son JW, Lee JH, Lee HW (2012) Extremely thin bilayer electrolyte for solid oxide fuel cells (SOFCs) fabricated by chemical solution deposition (CSD). Adv Mater 24:3373–3377
Minervini L, Zacate MO, Grimes RW (1999) Defect cluster formation in M2O3-doped CeO2. Solid State Ionics 116:339–349
Kharton VV, Marques FMB, Atkinson A (2004) Transport properties of solid oxide electrolyte ceramics: a brief review. Solid State Ionics 174:135–149
Serra JM, Vert VB, Buc¨hler O, Meulenberg WA, Buchkremer HP (2008) IT-SOFC supported on mixed oxygen ionic-electronic conducting composites. Chem Mater 20:3867–3875
Singhal SC (2000) Advances in solid oxide fuel cell technology. Solid State Ionics 135:305–313
Menzler NH, Tietz F, Uhlenbruck S, Buchkremer HP, Stöver D (2010) Materials and manufacturing technologies for solid oxide fuel cells. J Mater Sci 45:3109–3135
Anjaneya KC, Nayaka GP, Manjanna J, Govindaraj G, Ganesha KN (2013) Preparation and characterization of Ce1−xGdxO2−δ(x=0.1-0.3) as solid electrolyte for intermediate temperature SOFC. J Alloys Compd 578:53–59
Steele BCH (1994) Oxygen transport and exchange in oxide ceramics. J Power Sources 49:1–14
Muhammed Ali SA, Anwar M, Abdalla AM, Somalu MR, Muchta A (2017) Ce0.80Sm0.10Ba0.05Er0.05O2-δ multi-doped ceria electrolyte for intermediate temperature solid oxide fuel cells. Ceram Int 43:1265–1271
Kim J, Lee D (2002) The effect of multiple doping on electrical conductivity of gadolinia-doped ceria electrolyte. Korean J Chem Eng 19:421–424
Stojmenović M, Bošković S, Žunić M, Varela JA, Prekajski M, Matović B, Mentus S (2014) Electrical properties of multidoped ceria. Ceram Int 40:9285–9292
Wang FY, Chen S, Wang Q, Yu S, Cheng S (2004) Study on Gd and Mg co-doped ceria electrolyte for intermediate temperature solid oxide fuel cells. Catal Today 97:189–194
Kashyap D, Patro PK, Lenka RK, Mahata T, Sinha PK (2014) Effects of Gd and Sr co-doping in CeO2 for electrolyte application in solid oxide fuel cell (SOFC). Ceram Int 40:11869–11875
Dudek M, Rapacz-Kmita A, Mroczkowska M, Mosiałek M, Mordarski G (2010) Co-doped ceria-based solid solution in the CeO2-M2O3-CaO, M=Sm, Gd system. Electrochim Acta 55:4387–4394
Zheng YF, Shi Y, Gu HT, Gao L, Chen H, Guo L (2009) La and Ca co-doped ceria-based electrolyte materials for IT-SOFCs. Mater Res Bull 44:1717–1172
Avila-Paredes HJ, Kim S (2006) The effect of segregated transition metal ions on the grain boundary resistivity of gadolinium doped ceria: alteration of the space charge potential. Solid State Ionics 177:3075–3080
Yeh TH, Chou CC (2007) Ionic conductivity investigation in samarium and strontium co-doped ceria system. Phys Scr T129:303–307
Singh NK, Singh P, Singh MK, Kumar D, Parkash O (2011) Auto-combustion and synthesis of Ce0.85Gd0.15O1.925 for intermediate temperature solid oxide fuel cells electrolyte. Solid State Ionics 192:431–434
Shannon RD (1976) Revised effective ionic radii and systematic studies of interatomie distances in halides and chaleogenides. Acta Cryst 32:751–767
Hong SJ, Virkar AV (1995) Lattice parameters and densities of rare-earth oxide doped ceria electrolytes. J Am Ceram Soc 78:433–439
Jaiswal N, Upadhyay S, Kumar D, Parkash O (2013) Ionic conductivity investigation in lanthanum (La) and strontium (Sr) co-doped ceria system. J Power Sources 222:230–236
Yamamura H, Katoh E, Ichikawa M, Kakinuma K, Mori T, Haneda H (2000) Multiple doping effect on the electrical conductivity in the (Ce1-x-yLaxMy)O2-DELTA (M=Ca, Sr) system. Electrochemistry 68:455–459
Cioatera N, Parvulescu V, Rolle A, Vannier RN (2009) Effect of strontium addition on europium-doped ceria properties. Solid State Ionics 180:681–687
Kim S, Maier J (2002) On the conductivity mechanism of nanocrystalline ceria. J Electrochem Soc 149:73–83
Cotton FA, Wilkinson G (1999) Advanced inorganic chemistry, 5th edn. Wiley, New York
Pikalova EY, Maragou VI, Demina AN, Demin AK, Tsiakaras PE (2008) The effect of co-dopant addition on the properties of Ln0.2Ce0.8O2-δ(Ln=Gd, Sm, La) solid-state electrolyte. J Power Sources 181:199–206
Singh N, Singh NK, Parkash O, Kumar D (2012) Preparation and characterization of co-doped (Ce0.80La0.15Al0.05O1.90) and multiple-doped (Ce0.80Sm0.10Gd0.05Al0.05O1.90 and Ce0.80Gd0.10Sm0.05Al0.05O1.90) ceria. Ionics 18:473–478
Kashyap D, Patro PK, Lenka RK, Mahata T, Sinha PK (2014) Effects of Gd and Sr co-doping in CeO 2 for electrolyte application in solid oxide fuel cell (SOFC). Ceram Int 40:11869–11875
Zheng Y, Gu H, Chen H, Gao L, Zhu X, Guo L (2009) Effect of Sm and Mg co-doping on the properties of ceria-based electrolyte materials for IT-SOFCs. Mater Res Bull 44:775–779
Kahlaouin M, Chefi S, Inoubli A, Madani A, Chef C (2013) Synthesis and electrical properties of co-doping with La3+, Nd3+, Y3+and Eu3+ citric acid-nitrate prepared samarium-doped ceria ceramics. Ceram Int 39:3873–3879
Gan Y, Cheng J, Li M, Zhan H, Sun W (2015) Enhanced ceria based electrolytes by codoping samaria and scandia for intermediate temperature solid oxide fuel cells. Mater Chem Phys 163:279–285
Zhu K, Liu H, Zhu X, Liu Y, Yang W (2015) Enhanced performance of solid oxide fuel cells by introducing a transition layer between nanostructured cathode and electrolyte. Int. J Hydrogen Energy 40:501–508
Funding
This work was financially supported by the Natural Science Foundation of Fujian Province, China (Grant No. 2017J01686) and Key Laboratory of Eco-materials Advanced Technology (Fuzhou University), Fujian Province University (Grant No. STHJ-KF1707).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, J., Wu, K., Tu, T. et al. Preparation and properties of lanthanum (La) and indium (In) co-doped ceria system for IT-SOFC. Ionics 25, 1747–1757 (2019). https://doi.org/10.1007/s11581-018-2671-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-018-2671-7