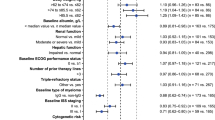
Abstract
Background
Teclistamab (JNJ-64007957), a B-cell maturation antigen × CD3 bispecific antibody, displayed potent T-cell–mediated cytotoxicity of multiple myeloma cells in preclinical studies.
Objective
A first-in-human, Phase I, dose escalation study (MajesTEC-1) is evaluating teclistamab in patients with relapsed/refractory multiple myeloma.
Patients and Methods
To estimate the efficacious therapeutic dosing range of teclistamab, pharmacokinetic (PK) data following the first cycle doses in the low-dose cohorts in the Phase I study were modeled using a 2-compartment model and simulated to predict the doses that would have average and trough serum teclistamab concentrations in the expected therapeutic range (between EC50 and EC90 values from an ex vivo cytotoxicity assay).
Results
The doses predicted to have average serum concentrations between the EC50 and EC90 range were validated. In addition, simulations showed that weekly intravenous and subcutaneous doses of 0.70 mg/kg and 0.72 mg/kg, respectively, resulted in mean trough levels comparable to the maximum EC90. The most active doses in the Phase I study were weekly intravenous doses of 0.27 and 0.72 mg/kg and weekly subcutaneous doses of 0.72 and 1.5 mg/kg, with the weekly 1.5 mg/kg subcutaneous doses selected as the recommended Phase II dose (RP2D). With active doses, exposure was maintained above the mean EC90. All patients who responded to the RP2D of teclistamab had exposure above the maximum EC90 in both serum and bone marrow on cycle 3, Day 1 of treatment.
Conclusions
Our findings show that PK simulations of early clinical data together with ex vivo cytotoxicity estimates can inform the identification of a bispecific antibody’s therapeutic range.
Clinical Trial Registration
NCT03145181, date of registration: May 9, 2017.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Early pharmacokinetic simulations using first-in-human Phase I study data and ex vivo cytotoxicity assay estimates provided guidance on the selection of a recommended Phase II dose (RP2D) of teclistamab. |
Active doses of teclistamab yielded drug exposure in the predicted therapeutic dosing range. |
All evaluable patients who responded to the RP2D of teclistamab had exposure above the predicted target concentration in both serum and bone marrow in cycle 3. |
1 Introduction
Multiple myeloma (MM), a plasma cell malignancy that is characterized by an excess of monoclonal immunoglobulin protein (or M protein), is estimated to represent ~ 10% of hematologic cancers in the USA [1]. Although therapeutic options have expanded substantially in the past decade, MM remains an incurable disease with limited options for patients who have relapsed or become refractory to standard therapies, i.e., proteasome inhibitors, immunomodulatory drugs, and monoclonal antibodies. Thus, identification and characterization of new therapeutic targets and treatment modalities are areas of active investigation.
B-cell maturation antigen (BCMA) is preferentially expressed on plasmablasts and differentiated plasma cells, with elevated expression reported in primary malignant plasma cells from patients with MM [2,3,4]. Moreover, BCMA levels were found to be higher in cultured bone marrow mononuclear cells and serum from patients with MM compared with healthy individuals [5]. Given these properties, BCMA has emerged as a target of considerable interest for new MM treatments; BCMA-targeting agents with different mechanisms of action have either been approved or are in clinical development for the treatment of MM, including antibody-drug conjugates, bispecific antibodies, and chimeric antigen receptor T-cell therapies [6].
Teclistamab (JNJ-64007957) is an off-the-shelf, BCMA × CD3 bispecific IgG4 antibody that redirects CD3+ T cells to induce cytotoxicity of BCMA-expressing MM cells. In ex vivo assays, teclistamab exhibited potent, T-cell–mediated cytotoxicity of MM cells derived from patients [4, 7]. The first-in-human, Phase I study (MajesTEC-1) of teclistamab monotherapy in patients with relapsed/refractory MM (RRMM) used a minimum anticipated biologic effect level (MABEL)-based starting dose [8], an approach consistent with guidelines from the US Food & Drug Administration (FDA) and the European Medicines Agency (EMA) for anticancer therapies with immune agonistic properties [9, 10]. The initial dose of teclistamab, 0.0003 mg/kg administered intravenously once every two weeks [8], was determined from the mean 20% of maximum effect (EC20) from an in vitro cytotoxicity assay using isolated T cells [4].
Analysis of preclinical data and first-in-human studies of 27 immune-activating agents [10] and a survey of the pharmaceutical industry [11] showed that MABEL-based starting doses frequently required numerous dose escalations before the optimal biologic dose, maximum tolerated dose, or recommended Phase II dose (RP2D) was reached. However, cytotoxicity assays may help to inform therapeutic concentrations. For example, retrospective analysis of the effective dose of the CD19 × CD3 bispecific antibody blinatumomab in patients with acute lymphoblastic leukemia was found to have an in vitro threshold of EC90 in the NALM-6 CD19+ human B-cell precursor leukemia cell line [12, 13]. Here, we prospectively coupled pharmacokinetic (PK) modeling of initial doses used in the first human study of teclistamab [8] with data from a patient-derived ex vivo cytotoxicity assay [4] to predict the efficacious therapeutic dosing range of teclistamab.
2 Methods
2.1 Teclistamab Ex Vivo T-Cell–Dependent Cytotoxicity Assay
As previously described [4], frozen purified bone marrow mononuclear cells from patients with MM were incubated for 48 hours with purified T cells from healthy donors (1:1) in the presence of increasing concentrations of teclistamab (0–532 nM) or control antibody. Depletion of live plasma cells was measured by detection of remaining CD138+ cells with a BD FACS Canto II cytometer. Mean (range) values for concentration at 50% of maximum effect (EC50) and concentration at 90% of maximum effect (EC90) for the cytotoxicity endpoint were estimated.
2.2 First-in-Human Phase I Study of Teclistamab
2.2.1 Study Design
Design details of the Phase I, first-in-human clinical study of teclistamab monotherapy (NCT03145181) have been described [8]. Eligible patients had MM per International Myeloma Working Group (IMWG) diagnostic criteria [14, 15] and were relapsed, refractory, or intolerant to established therapies. Primary objectives were to identify the RP2D and characterize safety and tolerability of teclistamab at the RP2D. Teclistamab was administered intravenously (range 0.0003–0.0192 mg/kg [every other week]; range 0.0192–0.720 mg/kg [weekly]) or subcutaneously (range 0.08–3.0 mg/kg weekly) in different cohorts, with step-up dosing used for ≥ 0.0384 mg/kg doses. In each teclistamab treatment cycle, investigators assessed response using 2011 IMWG response criteria [14, 15].
2.2.2 Pharmacokinetic Analyses
In weekly intravenous (IV) and subcutaneous (SC) dosing cohorts, blood samples were collected for the measurement of serum teclistamab concentrations on Days 1, 2, 3, 8, and 15 of cycles 1 and 3, on Days 1 and 15 of cycle 2, and on Day 1 of cycle 4; additional samples were collected on Days 4 and 6 of cycles 1 and 3 in the SC dosing cohorts. Serum samples were analyzed for teclistamab concentrations using a validated electrochemiluminescence-based immunoassay (ECLIA) format on the Meso Scale Discovery (MSD®) platform. Bone marrow aspirate samples were collected on Day 1 of cycle 3 for measurement of teclistamab concentrations, if feasible, and were also analyzed using the ECLIA assay.
2.3 Teclistamab Pharmacokinetic Simulations
A non-linear mixed-effect modeling approach was used for PK modeling and simulations. The PK data following the first cycle doses in the Phase I study low-dose cohorts were described with a two-compartment PK model with linear clearance. Pharmacokinetic simulations were performed using the estimated parameters to predict the doses that would yield trough serum teclistamab concentrations higher than the ex vivo EC90 value. Monolix2018 R1 (Lixosoft) was used to obtain estimates of model parameters, and R package mlxR (Lixosoft) was used for PK simulations. The goodness-of-fit was assessed by visual inspection, Akaike information criterion, Bayesian information criterion, and coefficient of variation of the estimated parameters.
2.4 Assessment of Teclistamab Concentrations in Cynomolgus Monkeys
In an exploratory study in male cynomolgus monkeys, IV teclistamab was given as either a single or repeat dose (total of five on Days 1, 8, 15, 22, and 29). Pharmacokinetic samples were collected up to Day 30 for animals administered weekly repeat doses (0.1 mg/kg, 1 mg/kg, and 10 mg/kg). Bone marrow was collected from each animal at necropsy (Day 30). Serum and bone marrow lysate samples were analyzed using ECLIA format on the MSD platform.
3 Results
3.1 Teclistamab Pharmacokinetic Simulations
Based on previously published results from an ex vivo T-cell–dependent cytotoxicity assay for teclistamab [4], reduction of MM cells was dose-dependent, and cytotoxicity was induced at a mean EC50 of 2.53 nM (range 1.47–4.21) and mean EC90 of 15.56 nM (range 1.71–41.29).
The teclistamab therapeutic range was estimated to be between the mean EC50 and maximum EC90 of the ex vivo cytotoxicity assay. Pharmacokinetic simulations were used to predict the doses that would have average and trough serum concentrations within the therapeutic range experimentally determined by the mean EC50 and maximum EC90 [4]. Weekly IV doses of 0.70 mg/kg and weekly SC doses of 0.72 mg/kg were predicted to have trough levels that reached the maximum EC90 (Fig. 1a, b).
Teclistamab pharmacokinetic simulation using a non-linear mixed-effect modeling approach. Simulated teclistamab serum concentrations following weekly a intravenous and b subcutaneous dosing. EC50 concentration at 50% of maximum effect, EC90 concentration at 90% of maximum effect, IV intravenous, Max maximum, SC subcutaneous
3.2 Teclistamab Pharmacokinetics Following the First Treatment Doses
Data from the Phase I study of teclistamab in patients with RRMM identified highly active doses as weekly IV doses of 0.27 and 0.72 mg/kg and weekly SC doses of 0.72 and 1.5 mg/kg (Fig. 2) [8]. Consistent with previously reported results across all dosing cohorts [8], maximum teclistamab concentrations occurred post-infusion and declined rapidly following the first full active IV doses (Fig. 3a), whereas teclistamab concentrations increased gradually and were sustained across the dosing interval following the first full active SC doses (Fig. 3b). Based on collective safety, efficacy, PK, and pharmacodynamic (PD) data, the RP2D was identified as a weekly SC dose of 1.5 mg/kg teclistamab [8]. At the RP2D, mean exposure exceeded the maximum EC90 from the ex vivo T-cell–dependent cytotoxicity assay [4] throughout the dosing interval.
Response rates at highly active doses of teclistamab [8]. CR complete response, ORR overall response rate, PR partial response, sCR stringent complete response, VGPR very good PR, ≥ VGPR VGPR or better. aIncludes stringent complete response, complete response, very good partial response, and partial response. Response data are based on a clinical cut-off date of March 29, 2021 [8]
Teclistamab pharmacokinetic profile following first treatment dose. Teclistamab serum concentrations after a most active intravenous doses and b most active subcutaneous doses. EC50 concentration at 50% of maximum effect, EC90 concentration at 90% of maximum effect, IV intravenous, Max maximum, SC subcutaneous
3.3 Teclistamab Concentrations in Serum and Bone Marrow
In cynomolgus monkeys, teclistamab concentrations were lower in bone marrow than in serum (Fig. 4a). The ratio of bone marrow to serum teclistamab concentration ranged from 0.3 to 0.6 in the tested doses (0.1 mg/kg, 1 mg/kg, and 10 mg/kg). In contrast, in most evaluable patients with RRMM who responded to teclistamab in the Phase I study, drug concentrations in bone marrow were comparable or higher than those in serum on cycle 3 Day 1 (Fig. 4b), which is in line with target-mediated drug disposition. All responders treated with weekly IV doses of 0.72 mg/kg and weekly SC doses of 0.72 and 1.5 mg/kg showed teclistamab serum concentration at or above the maximum EC90 [4] on cycle 3 Day 1; those treated at the RP2D had exposure above the maximum EC90 [4] in both serum and bone marrow (Fig. 4b).
Teclistamab concentrations in serum versus bone marrow. a Teclistamab serum concentrations in male cynomolgus monkeys and b teclistamab serum and bone marrow concentrations in patients with multiple myeloma treated with highly active intravenous and subcutaneous doses. Percentage of bone marrow plasma cells at baseline for each patient are shown. BMPC bone marrow plasma cell, EC90 concentration at 90% of maximum effect, IV intravenous, Max maximum, NR nonresponder, SC subcutaneous. aPatients had a partial response or better. bPatients had stable or progressive disease as best response
4 Discussion
In vitro and ex vivo studies provided a strong preclinical rationale to support testing of teclistamab in patients with MM [4, 7]. Data from in vitro analyses that demonstrated T-cell activation, T-cell–mediated cytotoxicity of BCMA-positive MM cells, and cytokine release [4] in response to teclistamab helped guide selection of the starting dose in the first-in-human MajesTEC-1 study using a conservative, MABEL-based approach. Based on the lowest mean EC20 from the most sensitive in vitro assay, the initial dose of teclistamab was identified as 0.0003 mg/kg administered intravenously once every two weeks.
It has been reported that MABEL-based starting doses are often substantially lower (i.e., by a factor of 100- to 1000-fold) than the dose that is subsequently deemed efficacious in patients [10, 11]. This can lead to delays in clinical development and access to potentially life-saving treatments, as well as increasing the number of patients who receive subtherapeutic doses. As a result, alternative strategies for selection of first-in-human starting doses are under consideration [11]. In the analyses reported here, we used a unique, prospective approach to predict the active doses of teclistamab that would have average and trough serum concentrations in the anticipated therapeutic dose range for patients with MM. Preclinical, ex vivo EC50 and EC90 estimates [4] were integrated with PK modeling and simulations of the early, low-dose cohorts. Our findings suggest that this approach could be valuable for prediction of efficacious doses of immuno-oncology drugs in general and could support acceleration of early clinical development.
In our model, responses were observed starting at an IV dose of 0.038 mg/kg, which was predicted to yield trough levels higher than the mean EC50 [8]. Additionally, PK modeling demonstrated that weekly IV doses of 0.27 mg/kg would yield trough levels between the mean and maximum EC90 and weekly SC doses of 0.72 mg/kg or higher would yield trough levels that approached or exceeded the maximum EC90 from the ex vivo cytotoxicity assay. In line with this prediction, weekly IV doses of 0.27 and 0.72 mg/kg and weekly SC doses of 0.72 and 1.5 mg/kg were highly active teclistamab doses in the Phase I study; overall response rates in these dose cohorts ranged from 60 to 75%. Thus, a PK modeling approach using preclinical and initial clinical data successfully predicted clinically active doses of teclistamab. While our approach identified clinical active doses of teclistamab, PK must be interpreted in the context of all clinical study data. A weekly SC teclistamab dose of 1.5 mg/kg was selected as the RP2D due to efficacy, safety, PK, and PD considerations [8]. While both 0.72 and 1.5 mg/kg SC doses were predicted to be within the therapeutic dosing range and yielded comparable response rates, the 1.5 mg/kg dose yielded durable responses. The 1.5 mg/kg dose level may be favorable due to the possibility of a low ratio of effector T cells to targets on tumor cells (E:T ratio) in bone marrow of some patients, such as those who have received multiple lines of bone marrow-depleting chemotherapy agents or those with a high burden of tumor cells [16, 17]. A limitation of our approach is that this model is dependent on the EC90 values derived from ex vivo assays, which do not account for intra-patient variability in E:T ratio, a covariate which may impact dose responses.
In addition to serum levels, we evaluated bone marrow concentrations of teclistamab in cynomolgus monkeys and in patients with MM from MajesTEC-1. In cynomolgus monkeys, the ratio of teclistamab concentration between bone marrow and serum ranged from 0.3 to 0.6 in the tested doses (0.1 mg/kg, 1 mg/kg, and 10 mg/kg), while in most patients with MM who responded to active teclistamab doses, teclistamab concentrations in bone marrow were comparable to or higher than those in serum. This finding may reflect the approximately 50-fold higher expression of BCMA that has been observed on MM cells in the bone marrow in patients with MM compared with healthy individuals [5].
In conclusion, PK modeling and simulation of early clinical data from the first-in-human MajesTEC-1 study, coupled with the ex vivo cytotoxicity estimates, provided guidance on active doses of teclistamab, including the RP2D. Active doses of teclistamab maintained exposure above the mean EC90 from the ex vivo cytotoxicity assay. In responders treated at the RP2D, exposure was sustained above the maximum EC90 in serum and bone marrow. The RP2D of teclistamab is under further exploration in an international, open-label, Phase II expansion study in patients with RRMM (NCT04557098). These findings suggest that preclinical, ex vivo cytotoxicity assays using patient bone marrow samples are an informative tool to predict the efficacious serum concentration of teclistamab. This approach may also be applied to other immuno-oncology drugs being evaluated for the treatment of MM or other hematologic malignancies.
Change history
01 August 2022
A Correction to this paper has been published: https://doi.org/10.1007/s11523-022-00904-y
References
Kumar SK, Rajkumar V, Kyle RA, van Duin M, Sonneveld P, Mateos MV, et al. Multiple myeloma. Nat Rev Dis Primers. 2017;3:17046. https://doi.org/10.1038/nrdp.2017.46.
Novak AJ, Darce JR, Arendt BK, Harder B, Henderson K, Kindsvogel W, et al. Expression of BCMA, TACI, and BAFF-R in multiple myeloma: a mechanism for growth and survival. Blood. 2004;103:689–94. https://doi.org/10.1182/blood-2003-06-2043.
Tai YT, Li XF, Breitkreutz I, Song W, Neri P, Catley L, et al. Role of B-cell-activating factor in adhesion and growth of human multiple myeloma cells in the bone marrow microenvironment. Cancer Res. 2006;66:6675–82. https://doi.org/10.1158/0008-5472.CAN-06-0190.
Pillarisetti K, Powers G, Luistro L, Babich A, Baldwin E, Li Y, et al. Teclistamab is an active T cell-redirecting bispecific antibody against B-cell maturation antigen for multiple myeloma. Blood Adv. 2020;4:4538–49. https://doi.org/10.1182/bloodadvances.2020002393.
Sanchez E, Li M, Kitto A, Li J, Wang CS, Kirk DT, et al. Serum B-cell maturation antigen is elevated in multiple myeloma and correlates with disease status and survival. Br J Haematol. 2012;158:727–38. https://doi.org/10.1111/j.1365-2141.2012.09241.x.
Shah N, Chari A, Scott E, Mezzi K, Usmani SZ. B-cell maturation antigen (BCMA) in multiple myeloma: rationale for targeting and current therapeutic approaches. Leukemia. 2020;34:985–1005. https://doi.org/10.1038/s41375-020-0734-z.
Frerichs KA, Broekmans MEC, Marin Soto JA, van Kessel B, Heymans MW, Holthof LC, et al. Preclinical activity of JNJ-7957, a novel BCMAxCD3 bispecific antibody for the treatment of multiple myeloma, is potentiated by daratumumab. Clin Cancer Res. 2020;26:2203–15. https://doi.org/10.1158/1078-0432.CCR-19-2299.
Usmani SZ, Garfall AL, van de Donk NWCJ, Nahi H, San-Miguel JF, Oriol A, et al. Teclistamab, a B-cell maturation antigen × CD3 bispecific antibody, in patients with relapsed or refractory multiple myeloma (MajesTEC-1): a multicentre, open-label, single-arm, phase 1 study. Lancet. 2021;398:665–74.
US Food & Drug Administration. Guidance for Industry S9 Nonclinical Evaluation for Anticancer Pharmaceuticals. https://www.fda.gov/media/73161/download. Accessed 21 May 2021.
Saber H, Gudi R, Manning M, Wearne E, Leighton JK. An FDA oncology analysis of immune activating products and first-in-human dose selection. Regul Toxicol Pharmacol. 2016;81:448–56. https://doi.org/10.1016/j.yrtph.2016.10.002.
Leach MW, Clarke DO, Dudal S, Han C, Li C, Yang Z, et al. Strategies and recommendations for using a data-driven and risk-based approach in the selection of first-in-human starting dose: an international consortium for innovation and quality in pharmaceutical development (IQ) assessment. Clin Pharmacol Ther. 2021;109:1395–415. https://doi.org/10.1002/cpt.2009.
Zhu M, Wu B, Brandl C, Johnson J, Wolf A, Chow A, et al. Blinatumomab, a bispecific T-cell Engager (BiTE((R))) for CD-19 targeted cancer immunotherapy: clinical pharmacology and its implications. Clin Pharmacokinet. 2016;55:1271–88. https://doi.org/10.1007/s40262-016-0405-4.
Yuraszeck T, Kasichayanula S, Benjamin JE. Translation and clinical development of bispecific T-cell engaging antibodies for cancer treatment. Clin Pharmacol Ther. 2017;101:634–45. https://doi.org/10.1002/cpt.651.
Rajkumar SV, Harousseau JL, Durie B, Anderson KC, Dimopoulos M, Kyle R, et al. Consensus recommendations for the uniform reporting of clinical trials: report of the International Myeloma Workshop Consensus Panel 1. Blood. 2011;117:4691–5. https://doi.org/10.1182/blood-2010-10-299487.
Durie BG, Miguel JF, Blade J, Rajkumar SV. Clarification of the definition of complete response in multiple myeloma. Leukemia. 2015;29:2416–7. https://doi.org/10.1038/leu.2015.290.
Duell J, Lammers PE, Djuretic I, Chunyk AG, Alekar S, Jacobs I, et al. Bispecific antibodies in the treatment of hematologic malignancies. Clin Pharmacol Ther. 2019;106:781–91. https://doi.org/10.1002/cpt.1396.
Trabolsi A, Arumov A, Schatz JH. T cell-activating bispecific antibodies in cancer therapy. J Immunol. 2019;203:585–92. https://doi.org/10.4049/jimmunol.1900496.
Acknowledgments
This study was funded by Janssen Research & Development, LLC. Medical writing support was provided by Joanna Bloom, PhD, and Valerie Kinchen, PhD, of Eloquent Scientific Solutions, and funded by Janssen Global Services, LLC. The authors thank the patients who participated in the study and their families and caregivers, the physicians and nurses who cared for patients and supported this clinical trial, the staff members at the study sites, and the staff members involved in data collection and analyses.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was funded by Janssen Research & Development.
Conflict of interest
B. Hanna, H. Adams, S. Shetty, and Y. Sun were employees of Johnson & Johnson during the completion of this work. All other authors are current employees of Johnson & Johnson and may hold stock in Johnson & Johnson.
Ethics approval
This clinical study was conducted in accordance with the Declaration of Helsinki and International Conference on Harmonisation guidelines for Good Clinical Practice. The clinical study protocol, amendments, and relevant documents were approved by an independent ethics committee/institutional review board at each study center. The cynomolgus monkey study did not require compliance with US FDA GLP regulations; however, this study was conducted using good scientific practices and following the applicable standard operating procedures of the testing facility and applicable test sites.
Consent to participate
All patients provided written informed consent prior to treatment.
Consent for publication
Not applicable.
Availability of data and material
The data sharing policy of Janssen Pharmaceutical Companies of Johnson & Johnson is available at https://www.janssen.com/clinical-trials/transparency. As noted on this site, requests for access to the study data can be submitted through Yale Open Data Access (YODA) Project site at http://yoda.yale.edu.
Code availability
Not applicable.
Author contributions
SG conceived and designed the study and analyzed and interpreted data. SXWL designed the study and analyzed and interpreted data. JS, JRI, JDG, and YE designed the clinical studies and provided study oversight. JDG, TS, AB, and B.W. Hilder managed the clinical study and interpreted results. KP, XM, SS, TYY, QJ, B. Hanna, and HA prepared materials, and collected analyzed and interpreted experimental data. YNS and AS interpreted the data. All authors critically reviewed and revised the manuscript and approved the final manuscript.
Additional information
The original article has been updated: Due to co-author name update.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Girgis, S., Lin, S.X.W., Pillarisetti, K. et al. Translational Modeling Predicts Efficacious Therapeutic Dosing Range of Teclistamab for Multiple Myeloma. Targ Oncol 17, 433–439 (2022). https://doi.org/10.1007/s11523-022-00893-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11523-022-00893-y