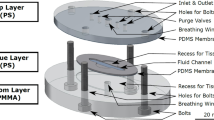
Abstract
The liver is one of the most important organs, with a complex physiology. Current in-vitro approaches are not accurate for disease modeling and drug toxicity research. One of those features is liver zonation, where cells display different physiological states due to different levels of oxygen and nutrient supplements. Organ-on-a-chip technology employs microfluidic platforms that enable a controlled environment for in-vitro cell culture. In this study, we propose a microfluidic design embedding a gas channel (of ambient air), creating an oxygen gradient. We numerically simulate different flow rates and cell densities with the COMSOL Multiphysics package considering cell-specific consumption rates of oxygen and glucose. We establish the cell density and flow rate for optimum oxygen and glucose distribution in the cell culture chamber. Furthermore, we show that a physiologically relevant concentration of oxygen and glucose in the chip is reached after 24 h and 30 min, respectively. The proposed microfluidic design and optimal parameters we identify in this paper provide a tool for in-vitro liver zonation studies. However, the microfluidic design is not exclusively for liver cell experiments but is foreseen to be applicable in cell studies where different gas concentration gradients are critical, e.g., studying hypoxia or toxic gas impact.
Graphical Abstract



Similar content being viewed by others
References
Juza RM, Pauli EM (2014) Clinical and surgical anatomy of the liver: a review for clinicians. Clin Anat 27(5):764–769. https://doi.org/10.1002/ca.22350
Wiśniewski JR, Vildhede A, Norén A, Artursson P (2016) In-depth quantitative analysis and comparison of the human hepatocyte and hepatoma cell line HepG2 proteomes. J Proteomics 136:234–247. https://doi.org/10.1016/j.jprot.2016.01.016
Kanabekova P, Kadyrova A, Kulsharova G (2022) Microfluidic organ-on-a-chip devices for liver disease modeling in vitro. Micromachines 13(3):428. https://doi.org/10.3390/mi13030428
Moradi E, Jalili-Firoozinezhad S, Solati-Hashjin M (2020) Microfluidic organ-on-a-chip models of human liver tissue. Acta Biomater 116:67–83. https://doi.org/10.1016/j.actbio.2020.08.041
Tomlinson L, Hyndman L, Firman JW, Bentley R, Kyffin JA, Webb SD, McGinty S, Sharma P (2019) In vitro liver zonation of primary rat hepatocytes. Front Bioeng Biotechnol 7:17. https://doi.org/10.3389/fbioe.2019.00017
Cunningham RP, Porat-Shliom N (2021) Liver zonation–revisiting old questions with new technologies. Front Physiol 12(2021):732929. https://doi.org/10.3389/fphys.2021.732929
Scheidecker B, Shinohara M, Sugimoto M, Danoy M, Nishikawa M, Sakai Y (2020) Induction of in vitro metabolic zonation in primary hepatocytes requires both near-physiological oxygen concentration and flux. Front Bioeng Biotechnol 8:524. https://doi.org/10.3389/fbioe.2020.00524
Kietzmann T (2019) Liver zonation in health and disease: hypoxia and hypoxia-inducible transcription factors as concert masters. Int J Mol Sci 20(9):2347. https://doi.org/10.3390/ijms20092347
Kietzmann T (2017) Metabolic zonation of the liver: the oxygen gradient revisited. Redox Biol 11:622–630. https://doi.org/10.1016/j.redox.2017.01.012
Li X, George SM, Vernetti L, Gough AH, Taylor DL (2018) A glass-based, continuously zonated and vascularized human liver acinus microphysiological system (vLAMPS) designed for experimental modeling of diseases and ADME/TOX. Lab Chip 18(17):2614–2631. https://doi.org/10.1039/C8LC00418H
Lee-Montiel FT, George SM, Gough AH, Sharma AD, Wu J, DeBiasio R, Vernetti LA, Taylor DL (2017) Control of oxygen tension recapitulates zone-specific functions in human liver microphysiology systems. Exp Biol Med 242(16):1617–1632. https://doi.org/10.1177/1535370217703978
Domansky K, Inman W, Serdy J, Dash A, Lim MH, Griffith LG (2010) Perfused multiwell plate for 3D liver tissue engineering. Lab Chip 10(1):51–58. https://doi.org/10.1039/B913221J
Tonon F, Giobbe GG, Zambon A, Luni C, Gagliano O, Floreani A, Grassi G, Elvassore N (2019) In vitro metabolic zonation through oxygen gradient on a chip. Sci Rep 9(1):1–10. https://doi.org/10.1038/s41598-019-49412-6
Chang C-W, Cheng Y-J, Tu M, Chen Y-H, Peng C-C, Liao W-H, Tung Y-C (2014) A polydimethylsiloxane–polycarbonate hybrid microfluidic device capable of generating perpendicular chemical and oxygen gradients for cell culture studies. Lab Chip 14(19):3762–3772. https://doi.org/10.1039/C4LC00732H
Lo JF, Sinkala E, Eddington DT (2010) Oxygen gradients for open well cellular cultures via microfluidic substrates. Lab Chip 10(18):2394–2401. https://doi.org/10.1039/C004660D
Tornberg K, Välimäki H, Valaskivi S, Mäki A-J, Jokinen M, Kreutzer J, Kallio P (2022) Compartmentalized organ-on-a-chip structure for spatiotemporal control of oxygen microenvironments. Biomed Microdevice 24(4):1–11. https://doi.org/10.1007/s10544-022-00634-y
Kang YBA, Eo J, Mert S, Yarmush ML, Usta OB (2018) Metabolic patterning on a chip: towards in vitro liver zonation of primary rat and human hepatocytes. Sci Rep 8(1):1–13. https://doi.org/10.1038/s41598-018-27179-6
Kang YB, Eo J, Bulutoglu B, Yarmush ML, Usta OB (2020) Progressive hypoxia-on-a-chip: An in vitro oxygen gradient model for capturing the effects of hypoxia on primary hepatocytes in health and disease. Biotechnol Bioeng 117(3):763–775. https://doi.org/10.1002/bit.27225
Han X, Zhu F, Chen L, Wu H, Wang T, Chen K (2020) Mechanism analysis of toxicity of sodium sulfite to human hepatocytes L02. Mol Cell Biochem 473(1):25–37. https://doi.org/10.1007/s11010-020-03805-8
Bulutoglu B, Rey-Bedón C, Kang YBA, Mert S, Yarmush ML, Usta OB (2019) A microfluidic patterned model of non-alcoholic fatty liver disease: applications to disease progression and zonation. Lab Chip 19(18):3022–3031. https://doi.org/10.1039/C9LC00354A
Wang H, Iovenitti P, Harvey E, Masood S (2002) Optimizing layout of obstacles for enhanced mixing in microchannels. Smart Mater Struct 11(5):662. https://doi.org/10.1088/0964-1726/11/5/306
Place TL, Domann FE, Case AJ (2017) Limitations of oxygen delivery to cells in culture: an underappreciated problem in basic and translational research. Free Radical Biol Med 113:311–322. https://doi.org/10.1016/j.freeradbiomed.2017.10.003
Shakeri A, Khan S, Didar TF (2021) Conventional and emerging strategies for the fabrication and functionalization of PDMS-based microfluidic devices. Lab Chip 21(16):3053–3075. https://doi.org/10.1039/D1LC00288K
Müller B, Sulzer P, Walch M, Zirath H, Buryška T, Rothbauer M, Ertl P, Mayr T (2021) Measurement of respiration and acidification rates of mammalian cells in thermoplastic microfluidic devices. Sens Actuators B: Chemical 334(2021):129664. https://doi.org/10.1016/j.snb.2021.129664
Carlborg CF, Haraldsson T, Öberg K, Malkoch M, Van Der Wijngaart W (2011) Beyond PDMS: off-stoichiometry thiol–ene (OSTE) based soft lithography for rapid prototyping of microfluidic devices. Lab Chip 11(18):3136–3147. https://doi.org/10.1039/C1LC20388F
Wölfle D, Schmidt H, Jungermann K (1983) Short-term modulation of glycogen metabolism, glycolysis and gluconeogenesis by physiological oxygen concentrations in hepatocyte cultures. Eur J Biochem 135(3):405–412. https://doi.org/10.1111/j.1432-1033.1983.tb07667.x
Goldstick T.K., Ciuryla V.T., Zuckerman L (1976) Diffusion of oxygen in plasma and blood, Oxygen transport to tissue—ii (1976) 183–190
Wagner BA, Venkataraman S, Buettner GR (2011) The rate of oxygen utilization by cells. Free Radical Biol Med 51(3):700–712. https://doi.org/10.1016/j.freeradbiomed.2011.05.024
Buchwald P (2009) FEM-based oxygen consumption and cell viability models for avascular pancreatic islets. Theor Biol Med Model 6(1):1–13. https://doi.org/10.1186/1742-4682-6-5
Markov DA, Lillie EM, Garbett SP, McCawley LJ (2014) Variation in diffusion of gases through PDMS due to plasma surface treatment and storage conditions. Biomed Microdevice 16(1):91–96. https://doi.org/10.1007/s10544-013-9808-2
Shiku H, Saito T, Wu C-C, Yasukawa T, Yokoo M, Abe H, Matsue T, Yamada H (2006) Oxygen permeability of surface-modified poly (dimethylsiloxane) characterized by scanning electrochemical microscopy. Chem Lett 35(2):234–235. https://doi.org/10.1246/cl.2006.234
Bavli D, Prill S, Ezra E, Levy G, Cohen M, Vinken M, Vanfleteren J, Jaeger M, Nahmias Y (2016) Real-time monitoring of metabolic function in liver-on-chip microdevices tracks the dynamics of mitochondrial dysfunction. Proc Natl Acad Sci 113(16):E2231–E2240. https://doi.org/10.1073/pnas.1522556113
Thermofischer Scientific Website (2023). https://www.thermofisher.com/order/catalog/product/11885084. Accessed 24 Jul 2023
Poon C (2022) Measuring the density and viscosity of culture media for optimized computational fluid dynamics analysis of in vitro devices, bioRxiv (2020) https://doi.org/10.1016/j.jmbbm.2021.105024
Miyamoto Y, Ikeuchi M, Noguchi H, Yagi T, Hayashi S (2015) Spheroid formation and evaluation of hepatic cells in a three-dimensional culture device. Cell Med 8(1–2):47–56
ATCC webpage (2023) <https://www.atcc.org/products/hb-8065>. Accessed 24 Jul 2023
Cogger VC, Hunt NJ, Le Couteur DG (2020) Fenestrations in the liver sinusoidal endothelial cell, The liver: biology and pathobiology (2020) 435–443 https://doi.org/10.1002/9781119436812.ch35
Reneman RS, Arts T, Hoeks AP (2006) Wall shear stress–an important determinant of endothelial cell function and structure–in the arterial system in vivo. J Vasc Res 43(3):251–269. https://doi.org/10.1159/000091648
Tilles AW, Baskaran H, Roy P, Yarmush ML, Toner M (2001) Effects of oxygenation and flow on the viability and function of rat hepatocytes cocultured in a microchannel flat-plate bioreactor. Biotechnol Bioeng 73(5):379–389. https://doi.org/10.1002/bit.1071
Tanaka Y, Yamato M, Okano T, Kitamori T, Sato K (2006) Evaluation of effects of shear stress on hepatocytes by a microchip-based system. Meas Sci Technol 17(12):3167. https://doi.org/10.1088/0957-0233/17/12/S08
Li W, Li P, Li N, Du Y, Lü S, Elad D, Long M (2021) Matrix stiffness and shear stresses modulate hepatocyte functions in a fibrotic liver sinusoidal model. Am J Physiol Gastrointest Liver Physiol 320(3):G272–G282. https://doi.org/10.1152/ajpgi.00379.2019
Lalor P, Adams D (1999) Adhesion of lymphocytes to hepatic endothelium. Mol Pathol 52(4):214. https://doi.org/10.1136/mp.52.4.214
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors have no conflict of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mahdavi, R., Hashemi-Najafabadi, S., Ghiass, M.A. et al. Microfluidic design for in-vitro liver zonation—a numerical analysis using COMSOL Multiphysics. Med Biol Eng Comput 62, 121–133 (2024). https://doi.org/10.1007/s11517-023-02936-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11517-023-02936-6