Abstract
Existing in silico models for single cell mechanics feature limited representations of cytoskeletal structures that contribute substantially to the mechanics of a cell. We propose a micromechanical hierarchical approach to capture the mechanical contribution of actin stress fibres. For a cell-specific fibroblast geometry with membrane, cytoplasm and nucleus, the Mori-Tanaka homogenization method was employed to describe cytoplasmic inhomogeneities and constitutive contribution of actin stress fibres. The homogenization was implemented in a finite element model of the fibroblast attached to a substrate through focal adhesions. Strain in cell membrane, cytoplasm and nucleus due to uniaxial substrate stretch was assessed for different stress fibre volume fractions and different elastic modulus of the substrate. A considerable decrease of the peak strain with increasing stress fibre content was observed in cytoplasm and nucleus but not the membrane, whereas the peak strain in cytoplasm, nucleus and membrane increased for increasing elastic modulus of the substrate.
Graphical abstract

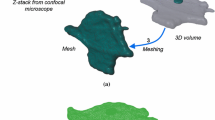
Finite element mesh of reconstructed human fibroblast and intracellular strain distribution in cell subjected to substrate stretch.









Similar content being viewed by others
Data availability
Abaqus input files of the finite element models used in this study are available on ZivaHUB (https://doi.org/10.25375/uct.9782798).
References
Huang H, Kamm RD, Lee RT (2004) Cell mechanics and mechanotransduction: pathways, probes, and physiology. Am J Phys Cell Phys 287(1):C1–C11
Brown TD (2000) Techniques for mechanical stimulation of cells in vitro: a review. J Biomech 33(1):3–14
Wang N, Naruse K, Stamenović D, Fredberg JJ, Mijailovich SM, Tolić-Nørrelykke IM et al (2001) Mechanical behavior in living cells consistent with the tensegrity model. Proc Natl Acad Sci 98(14):7765–7770
Moeendarbary E, Harris AR (2014) Cell mechanics: principles, practices, and prospects. Wiley. Interdisciplinary Reviews: Systems Biology and Medicine 6(5):371–388
Hu S, Chen J, Fabry B, Numaguchi Y, Gouldstone A, Ingber DE et al (2003) Intracellular stress tomography reveals stress focusing and structural anisotropy in cytoskeleton of living cells. Am J Phys Cell Phys 285(5):C1082–C1090
Karcher H, Lammerding J, Huang H, Lee RT, Kamm RD, Kaazempur-Mofrad MR (2003) A three-dimensional viscoelastic model for cell deformation with experimental verification. Biophys J 85(5):3336–3349
Ohayon J, Tracqui P (2005) Computation of adherent cell elasticity for critical cell-bead geometry in magnetic twisting experiments. Ann Biomed Eng 33(2):131–141
Evans ND, Gentleman E (2014) The role of material structure and mechanical properties in cell–matrix interactions. J Mater Chem B 2(17):2345–2356
Hochmuth RM (2000) Micropipette aspiration of living cells. J Biomech 33:15–22
Maksym GN, Fabry B, Butler JP, Navajas D, Tschumperlin DJ, Laporte JD, Fredberg JJ (2000) Mechanical properties of cultured human airway smooth muscle cells from 0.05 to 0.4 Hz. J Appl Physiol 89(4):1619–1632
Roduit C, Sekatski S, Dietler G, Catsicas S, Lafont F, Kasas S (2009) Stiffness tomography by atomic force microscopy. Biophys J 97(2):674–677
Thomas G, Burnham NA, Camesano TA, Wen Q (2013) Measuring the mechanical properties of living cells using atomic force microscopy. J Vis Exp 27(76):e50497
Sosa MS, Bragado P, Aguirre-Ghiso JA (2014) Mechanisms of disseminated cancer cell dormancy: an awakening field. Nat Rev Cancer 14(9):611–622
Mavrakis M, Azou-Gros Y, Tsai FC, Alvarado J, Bertin A, Iv F, Kress A, Brasselet S, Koenderink GH, Lecuit T (2014) Septins promote F-actin ring formation by crosslinking actin filaments into curved bundles. Nat Cell Biol 16(4):322–334
Lancaster OM, Baum B (2014) Shaping up to divide: coordinating actin and microtubule cytoskeletal remodelling during mitosis. In Seminars in cell & developmental biology 34:109–115
Stewart MP, Toyoda Y, Hyman AA, Müller DJ (2012) Tracking mechanics and volume of globular cells with atomic force microscopy using a constant-height clamp. Nat Protoc 7(1):143–154
Park CY, Tambe D, Alencar AM, Trepat X, Zhou EH, Millet E, Butler JP, Fredberg JJ (2010) Mapping the cytoskeletal prestress. Am J Phys Cell Phys 298(5):C1245–C1252
Rotsch C, Radmacher M (2000) Drug-induced changes of cytoskeletal structure and mechanics in fibroblasts: an atomic force microscopy study. Biophys J 78(1):520–535
Fletcher DA, Mullins RD (2010) Cell mechanics and the cytoskeleton. Nature 463(7280):485–492
Wu HW, Kuhn T, Moy VT (1998) Mechanical properties of L929 cells measured by atomic force microscopy: effects of anticytoskeletal drugs and membrane crosslinking. Scanning: The Journal of Scanning Microscopies 20(5):389–397
Janmey PA, Miller RT (2011) Mechanisms of mechanical signaling in development and disease. J Cell Sci 124(1):9–18
Lekka M, Laidler P, Gil D, Lekki J, Stachura Z, Hrynkiewicz AZ (1999) Elasticity of normal and cancerous human bladder cells studied by scanning force microscopy. Eur Biophys J 28(4):312–316
Na S, Sun Z, Meininger GA, Humphrey JD (2004) On atomic force microscopy and the constitutive behavior of living cells. Biomech Model Mechanobiol 3(2):75–84
Humphrey JD (2002) On mechanical modeling of dynamic changes in the structure and properties of adherent cells. Mathematics and Mechanics of Solids 7(5):521–539
Lim CT, Zhou EH, Quek ST (2006) Mechanical models for living cells-a review. J Biomech 39(2):195–216
Lavagnino M, Arnoczky SP, Kepich E, Caballero O, Haut RC (2008) A finite element model predicts the mechanotransduction response of tendon cells to cyclic tensile loading. Biomech Model Mechanobiol 7(5):405–416
Miller P, Hu L, Wang J (2010) Finite element simulation of cell–substrate decohesion by laser-induced stress waves. J Mech Behav Biomed Mater 3(3):268–277
Abdalrahman T, Dubuis L, Green J, Davies N, Franz T (2017) Cellular mechanosensitivity to substrate stiffness decreases with increasing dissimilarity to cell stiffness. Biomech Model Mechanobiol 16(6):2063–2075
Simon BR, Kaufmann MV, McAfee MA, Baldwin AL (1993) Finite element models for arterial wall mechanics. J Biomech Eng 115:489–496
Milner JS, Grol MW, Beaucage KL, Dixon SJ, Holdsworth DW (2012) Finite-element modeling of viscoelastic cells during high-frequency cyclic strain. Journal of functional biomaterials 3(1):209–224
Mullen CA, Vaughan TJ, Voisin MC, Brennan MA, Layrolle P, McNamara LM (2014) Cell morphology and focal adhesion location alters internal cell stress. J R Soc Interface 11(101):20140885
Barreto S, Clausen CH, Perrault CM, Fletcher DA, Lacroix D (2013) A multi-structural single cell model of force-induced interactions of cytoskeletal components. Biomaterials 34(26):6119–6126
Barreto S, Perrault CM, Lacroix D (2014) Structural finite element analysis to explain cell mechanics variability. J Mech Behav Biomed Mater 38:219–231
Slomka N, Gefen A (2012) Relationship between strain levels and permeability of the plasma membrane in statically stretched myoblasts. Ann Biomed Eng 40(3):606–618
Yao Y, Lacroix D, Mak AF (2016) Effects of oxidative stress-induced changes in the actin cytoskeletal structure on myoblast damage under compressive stress: confocal-based cell-specific finite element analysis. Biomech Model Mechanobiol 15(6):1495–1508
Chen A, Moy VT (2000) Cross-linking of cell surface receptors enhances cooperativity of molecular adhesion. Biophys J 78(6):2814–2820
Berg JM, Tymoczko JL, Stryer L (2002) Biochemistry, WH Freeman & Co. New York.
Fortino S, Zagari G, Mendicino AL, Dill-Langer G (2012) A simple approach for FEM simulation of mode I cohesive crack growth in glued laminated timber under short-term loading. Journal of Structural Mechanics 45(1):1–20
Bartalena G, Loosli Y, Zambelli T, Snedeker JG (2012) Biomaterial surface modifications can dominate cell–substrate mechanics: the impact of PDMS plasma treatment on a quantitative assay of cell stiffness. Soft Matter 8(3):673–681
Balaban NQ, Schwarz US, Riveline D, Goichberg P, Tzur G, Sabanay I, Mahalu D, Safran S, Bershadsky A, Addadi L, Geiger B (2001) Force and focal adhesion assembly: a close relationship studied using elastic micropatterned substrates. Nat Cell Biol 3(5):466–472
Goffin JM, Pittet P, Csucs G, Lussi JW, Meister JJ, Hinz B (2006) Focal adhesion size controls tension-dependent recruitment of α-smooth muscle actin to stress fibers. J Cell Biol 172(2):259–268
Park K, Paulino GH (2012) Computational implementation of the PPR potential-based cohesive model in ABAQUS: educational perspective. Eng Fract Mech 93:239–262
Dávila CG, Camanho PP, Turon A (2008) Effective simulation of delamination in aeronautical structures using shells and cohesive elements. J Aircr 45(2):663–672
Breuls RG, Sengers BG, Oomens CW, Bouten CV, Baaijens FP (2002) Predicting local cell deformations in engineered tissue constructs: a multilevel finite element approach. J Biomech Eng 124(2):198–207
Baaijens FP, Trickey WR, Laursen TA, Guilak F (2005) Large deformation finite element analysis of micropipette aspiration to determine the mechanical properties of the chondrocyte. Ann Biomed Eng 33(4):494–501
Jean RP, Chen CS, Spector AA (2005) Finite-element analysis of the adhesion-cytoskeleton-nucleus mechanotransduction pathway during endothelial cell rounding: axisymmetric model. J Biomech Eng 127(4):594–600
Hochmuth RM, Mohandas N, Blackshear PL Jr (1973) Measurement of the elastic modulus for red cell membrane using a fluid mechanical technique. Biophys J 13(8):747–762
Lelidis I, Joanny JF (2013) Interaction of focal adhesions mediated by the substrate elasticity. Soft Matter 9(46):11120–11128
Nicolas A, Safran SA (2006) Limitation of cell adhesion by the elasticity of the extracellular matrix. Biophys J 91(1):61–73
Wang N, Stamenovic D (2000) Contribution of intermediate filaments to cell stiffness, stiffening, and growth. Am J Phys Cell Phys 279(1):C188–C194
Brangwynne CP, MacKintosh FC, Kumar S, Geisse NA, Talbot J, Mahadevan L, Parker KK, Ingber DE, Weitz DA (2006) Microtubules can bear enhanced compressive loads in living cells because of lateral reinforcement. J Cell Biol 173(5):733–741
Heidemann SR, Wirtz D (2004) Towards a regional approach to cell mechanics. Trends Cell Biol 14(4):160–166
Feneberg W, Aepfelbacher M, Sackmann E (2004) Microviscoelasticity of the apical cell surface of human umbilical vein endothelial cells (HUVEC) within confluent monolayers. Biophys J 87(2):1338–1350
Bakkali A, Azrar L, Fakri N (2011) Modeling of effective properties of multiphase magnetoelectroelastic heterogeneous materials. Computers, Materials, & Continua 23(3):201–231
Ryu S, Lee S, Jung J, Lee J, Kim Y (2019) Micromechanics-based homogenization of the effective physical properties of composites with an anisotropic matrix and interfacial imperfections. Frontiers in Materials 6:21
Eshelby JD (1957) The determination of the elastic field of an ellipsoidal inclusion, and related problems. Proceedings of the royal society of London. Series A Mathematical and physical sciences 241(1226):376–396
Mori T, Tanaka K (1973) Average stress in matrix and average elastic energy of materials with misfitting inclusions. Acta Metall 21(5):571–574
Ingber DE, Heidemann SR, Lamoureux P, Buxbaum RE (2000) Opposing views on tensegrity as a structural framework for understanding cell mechanics. J Appl Physiol 89:1663–1678
Satcher RL Jr, Dewey CF Jr (1996) Theoretical estimates of mechanical properties of the endothelial cell cytoskeleton. Biophys J 71(1):109–118
Kohn JC, Abdalrahman T, Sack KL, Reinhart-King CA, Franz T (2018) Endothelial cells on an aged subendothelial matrix display heterogeneous strain profiles in silico. Biomech Model Mechanobiol 17(5):1405–1414
Kohn JC, Abdalrahman T, Sack KL, Reinhart-King CA, Franz T (2019) Cell focal adhesion clustering leads to decreased and homogenized basal strains. International journal for numerical methods in biomedical engineering 35(12):e3260
Dahl KN, Ribeiro AJ, Lammerding J (2008) Nuclear shape, mechanics, and mechanotransduction. Circ Res 102(11):1307–1318
Petroll WM, Cavanagh HD, Barry P, Andrews P, Jester JV (1993) Quantitative analysis of stress fiber orientation during corneal wound contraction. J Cell Sci 104(2):353–363
Sbrana F, Sassoli C, Meacci E, Nosi D, Squecco R, Paternostro F, Tiribilli B, Zecchi-Orlandini S, Francini F, Formigli L (2008) Role for stress fiber contraction in surface tension development and stretch-activated channel regulation in C2C12 myoblasts. Am J Phys Cell Phys 295(1):C160–C172
Caille N, Thoumine O, Tardy Y, Meister JJ (2002) Contribution of the nucleus to the mechanical properties of endothelial cells. J Biomech 35(2):177–187
Ferko MC, Bhatnagar A, Garcia MB, Butler PJ (2007) Finite-element stress analysis of a multicomponent model of sheared and focally-adhered endothelial cells. Ann Biomed Eng 35(2):208–223
Gilchrist CL, Witvoet-Braam SW, Guilak F, Setton LA (2007) Measurement of intracellular strain on deformable substrates with texture correlation. J Biomech 40(4):786–794
Schaffer JL, Rizen M, L'Italien GJ, Benbrahim A, Megerman J, Gerstenfeld LC, Gray ML (1994) Device for the application of a dynamic biaxially uniform and isotropic strain to a flexible cell culture membrane. J Orthop Res 12(5):709–719
Park JS, Chu JS, Cheng C, Chen F, Chen D, Li S (2004) Differential effects of equiaxial and uniaxial strain on mesenchymal stem cells. Biotechnol Bioeng 88(3):359–368
Wang N (2010) Structural basis of stress concentration in the cytoskeleton. Molecular & Cellular Biomechanics 7(1):33–44
Gavara N, Chadwick RS (2016) Relationship between cell stiffness and stress fiber amount assessed by simultaneous atomic force microscopy and live-cell fluorescence imaging. Biomech Model Mechanobiol 15(3):511–523. https://doi.org/10.1007/s10237-015-0706-9
Peeters EA, Oomens CW, Bouten CV, Bader DL, Baaijens FP (2005) Mechanical and failure properties of single attached cells under compression. J Biomech 38(8):1685–1693. https://doi.org/10.1016/j.jbiomech.2004.07.018
Funding
The research reported in this publication was supported by the National Research Foundation of South Africa (UID 92531 and 93542) and the South African Medical Research Council (SIR 328148).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Disclaimer
Views and opinions expressed are not those of the NRF or MRC but the authors.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 14 kb)
Rights and permissions
About this article
Cite this article
Abdalrahman, T., Davies, N.H. & Franz, T. In silico stress fibre content affects peak strain in cytoplasm and nucleus but not in the membrane for uniaxial substrate stretch. Med Biol Eng Comput 59, 1933–1944 (2021). https://doi.org/10.1007/s11517-021-02393-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11517-021-02393-z