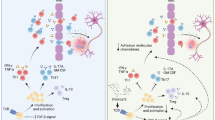
Abstract
Neuroinflammation leads to tissue injury causing many of the clinical symptoms of Multiple Sclerosis, an autoimmune disorder of the central nervous system (CNS). While T cells, specifically Th1 and Th17 cells, are the ultimate effectors of this disease, dendritic cells (DCs) mediate T cell polarization, activation, etc. In our previous study, Apigenin, a natural flavonoid, has been shown to reduce EAE disease severity through amelioration of demyelination in the CNS as well as the sequestering of DCs and other myeloid cells in the periphery. Here, we show that Apigenin exerts its effects possibly through shifting DC modulated T cell responses from Th1 and Th17 type towards Treg directed responses evident through the decrease in T-bet, IFN-γ (Th1), IL-17 (Th17) and increase in IL-10, TGF-β and FoxP3 (Treg) expression in cells from both normal human donors and EAE mice. RelB, an NF-κβ pathway protein is central to DC maturation, its antigen presentation capabilities and DC-mediated T cell activation. Apigenin reduced mRNA and protein levels of RelB and also reduced its nuclear translocation. Additionally, siRNA-mediated silencing of RelB further potentiated the RelB-mediated effects of Apigenin thus confirming its role in Apigenin directed regulation of DC biology. These results provide key information about the molecular events controlled by Apigenin in its regulation of DC activity marking its potential as a therapy for neuroinflammatory disease.

Graphical Abstract












Similar content being viewed by others
Abbreviations
- BBB:
-
Blood-brain barrier
- TNF-α:
-
Tumor necrosis factor
- IFN-γ:
-
Interferon-γ
- NF-κB:
-
Nuclear factor kappa B
- MS:
-
Multiple Sclerosis
- CNS:
-
Central nervous system
- EAE:
-
Experimental autoimmune encephalomyelitis
- Treg :
-
T regulatory cell
- DC:
-
Dendritic cells
- PBMC:
-
Peripheral blood mononuclear cell
- PBL:
-
Peripheral blood lymphocyte
- LPS:
-
Lipopolysaccharide
References
Allen JE, Wynn TA (2011) Evolution of Th2 immunity: a rapid repair response to tissue destructive pathogens. PLoS Pathog 7:e1002003. https://doi.org/10.1371/journal.ppat.1002003
Bailey SL, Schreiner B, McMahon EJ, Miller SD (2007) CNS myeloid DCs presenting endogenous myelin peptides 'preferentially' polarize CD4+ T(H)-17 cells in relapsing EAE. Nature immunology 8:172–180. https://doi.org/10.1038/ni1430
Bearoff F et al (2016) Natural genetic variation profoundly regulates gene expression in immune cells and dictates susceptibility to CNS autoimmunity genes. Immun 17:386–395. https://doi.org/10.1038/gene.2016.37
Becher B, Spath S, Goverman J (2017) Cytokine networks in neuroinflammation. Nat Rev Immunol 17:49–59. https://doi.org/10.1038/nri.2016.123
Blanco P, Palucka AK, Pascual V, Banchereau J (2008) Dendritic cells and cytokines in human inflammatory and autoimmune diseases. Cytokine Growth Factor Rev 19:41–52. https://doi.org/10.1016/j.cytogfr.2007.10.004
Bruno A et al (2011) Apigenin affects leptin/leptin receptor pathway and induces cell apoptosis in lung adenocarcinoma cell line. Eur J Cancer 47:2042–2051. https://doi.org/10.1016/j.ejca.2011.03.034
Byun S et al (2013) Src kinase is a direct target of apigenin against UVB-induced skin inflammation. Carcinogenesis 34:397–405. https://doi.org/10.1093/carcin/bgs358
Cardenas H, Arango D, Nicholas C, Duarte S, Nuovo GJ, He W, Voss OH, Gonzalez-Mejia ME, Guttridge DC, Grotewold E, Doseff AI (2016) Dietary Apigenin exerts immune-regulatory activity in vivo by reducing NF-kappaB activity, Halting Leukocyte Infiltration and Restoring Normal Metabolic Function. Int J Mol Sci 17:323. https://doi.org/10.3390/ijms17030323
Cejas PJ et al (2005) Regulation of RelB expression during the initiation of dendritic cell differentiation. Mol Cell Biol 25:7900–7916. https://doi.org/10.1128/MCB.25.17.7900-7916.2005
Chuang CM, Monie A, Wu A, Hung CF (2009) Combination of apigenin treatment with therapeutic HPV DNA vaccination generates enhanced therapeutic antitumor effects. Journal of biomedical science 16:49. https://doi.org/10.1186/1423-0127-16-49
Colton CA (2013) Immune heterogeneity in neuroinflammation: dendritic cells in the brain. J NeuroImmune Pharmacol 8:145–162. https://doi.org/10.1007/s11481-012-9414-8
Czeczot H, Tudek B, Kusztelak J, Szymczyk T, Dobrowolska B, Glinkowska G, Malinowski J, Strzelecka H (1990) Isolation and studies of the mutagenic activity in the Ames test of flavonoids naturally occurring in medical herbs. Mutat Res 240:209–216
Funakoshi-Tago M, Nakamura K, Tago K, Mashino T, Kasahara T (2011) Anti-inflammatory activity of structurally related flavonoids, Apigenin, Luteolin and Fisetin. Int Immunopharmacol 11:1150–1159. https://doi.org/10.1016/j.intimp.2011.03.012
Gasparini C, Foxwell BM, Feldmann M (2013) RelB/p50 regulates TNF production in LPS-stimulated dendritic cells and macrophages. cytokine 61:736–740. https://doi.org/10.1016/j.cyto.2012.12.029
Gentile D, Fornai M, Colucci R, Pellegrini C, Tirotta E, Benvenuti L, Segnani C, Ippolito C, Duranti E, Virdis A, Carpi S, Nieri P, Németh ZH, Pistelli L, Bernardini N, Blandizzi C, Antonioli L (2018) The flavonoid compound apigenin prevents colonic inflammation and motor dysfunctions associated with high fat diet-induced obesity. PLoS One 13:e0195502. https://doi.org/10.1371/journal.pone.0195502
Ginwala R, McTish E, Raman C, Singh N, Nagarkatti M, Nagarkatti P, Sagar D, Jain P, Khan ZK (2016) Apigenin, a Natural Flavonoid, Attenuates EAE Severity Through the Modulation of Dendritic Cell and Other Immune Cell Functions. J Neuroimmune Pharmacol 11:36–47. https://doi.org/10.1007/s11481-015-9617-x
Greter M et al (2005) Dendritic cells permit immune invasion of the CNS in an animal model of multiple sclerosis. Nature medicine 11:328–334. https://doi.org/10.1038/nm1197
Hofmann J, Mair F, Greter M, Schmidt-Supprian M, Becher B (2011) NIK signaling in dendritic cells but not in T cells is required for the development of effector T cells and cell-mediated immune responses. J Exp Med 208:1917–1929. https://doi.org/10.1084/jem.20110128
Isaksson M, Ardesjo B, Ronnblom L, Kampe O, Lassmann H, Eloranta ML, Lobell A (2009) Plasmacytoid DC promote priming of autoimmune Th17 cells and EAE. Eur J Immunol 39:2925–2935. https://doi.org/10.1002/eji.200839179
Jain P, Ahuja J, Khan ZK, Shimizu S, Meucci O, Jennings SR, Wigdahl B (2007) Modulation of dendritic cell maturation and function by the tax protein of human T cell leukemia virus type 1. J Leukoc Biol 82:44–56. https://doi.org/10.1189/jlb.1006641
Jain P, Coisne C, Enzmann G, Rottapel R, Engelhardt B (2010) Alpha4beta1 integrin mediates the recruitment of immature dendritic cells across the blood-brain barrier during experimental autoimmune encephalomyelitis. J Immunol 184:7196–7206. https://doi.org/10.4049/jimmunol.0901404
Jensen SS, Gad M (2010) Differential induction of inflammatory cytokines by dendritic cells treated with novel TLR-agonist and cytokine based cocktails: targeting dendritic cells in autoimmunity. J Inflamm (Lond) 7:37. https://doi.org/10.1186/1476-9255-7-37
Jeong GS, Lee SH, Jeong SN, Kim YC, Kim EC (2009) Anti-inflammatory effects of apigenin on nicotine- and lipopolysaccharide-stimulated human periodontal ligament cells via heme oxygenase-1. Int Immunopharmacol 9:1374–1380. https://doi.org/10.1016/j.intimp.2009.08.015
Jung UJ, Cho YY, Choi MS (2016) Apigenin ameliorates dyslipidemia, hepatic steatosis and insulin resistance by modulating metabolic and transcriptional profiles in the liver of high-fat diet-induced obese mice. Nutrients 8 doi:https://doi.org/10.3390/nu8050305
Karamese M, Erol HS, Albayrak M, Findik Guvendi G, Aydin E, Aksak Karamese S (2016) Anti-oxidant and anti-inflammatory effects of apigenin in a rat model of sepsis: an immunological, biochemical, and histopathological study. Immunopharmacol Immunotoxicol 38:228–237. https://doi.org/10.3109/08923973.2016.1173058
Kemanetzoglou E, Andreadou E (2017) CNS Demyelination with TNF-alpha Blockers. Curr Neurol Neurosci Rep 17:36. https://doi.org/10.1007/s11910-017-0742-1
Krausgruber T, Saliba D, Ryzhakov G, Lanfrancotti A, Blazek K, Udalova IA (2010) IRF5 is required for late-phase TNF secretion by human dendritic cells. Blood 115:4421–4430. https://doi.org/10.1182/blood-2010-01-263020
Krementsov DN, Noubade R, Dragon JA, Otsu K, Rincon M, Teuscher C (2014) Sex-specific control of central nervous system autoimmunity by p38 mitogen-activated protein kinase signaling in myeloid cells. Ann Neurol 75:50–66. https://doi.org/10.1002/ana.24020
Kumar KS, Sabu V, Sindhu G, Rauf AA, Helen A (2018) Isolation, identification and characterization of apigenin from Justicia gendarussa and its anti-inflammatory activity. Int Immunopharmacol 59:157–167. https://doi.org/10.1016/j.intimp.2018.04.004
Kumar S, Pandey AK (2013) Chemistry and biological activities of flavonoids: an overview. Scientific World Journal 2013:162750. https://doi.org/10.1155/2013/162750
Kumazawa Y, Kawaguchi K, Takimoto H (2006) Immunomodulating effects of flavonoids on acute and chronic inflammatory responses caused by tumor necrosis factor alpha. Curr Pharm Des 12:4271–4279
Lee JH, Zhou HY, Cho SY, Kim YS, Lee YS, Jeong CS (2007) Anti-inflammatory mechanisms of apigenin: inhibition of cyclooxygenase-2 expression, adhesion of monocytes to human umbilical vein endothelial cells, and expression of cellular adhesion molecules. Arch Pharm Res 30:1318–1327
Leyva-Lopez N, Gutierrez-Grijalva EP, Ambriz-Perez DL, Heredia JB (2016) Flavonoids as cytokine modulators: a possible therapy for inflammation-related diseases. Int J Mol Sci 17. doi:https://doi.org/10.3390/ijms17060921
Li M et al (2007) Immune modulation and tolerance induction by RelB-silenced dendritic cells through RNA interference. J Immunol 178:5480–5487
Li X, Han Y, Zhou Q, Jie H, He Y, Han J, He J, Jiang Y, Sun E (2016) Apigenin, a potent suppressor of dendritic cell maturation and migration, protects against collagen-induced arthritis. J Cell Mol Med 20:170–180. https://doi.org/10.1111/jcmm.12717
Ludewig P et al (2016) Dendritic cells in brain diseases. Biochim Biophys Acta 1862:352–367. https://doi.org/10.1016/j.bbadis.2015.11.003
Lüssi F, Zipp F, Witsch E (2016) Dendritic cells as therapeutic targets in neuroinflammation. Cell Mol Life Sci 73:2425–2450. https://doi.org/10.1007/s00018-016-2170-9
Maldonado RA, von Andrian UH (2010) How tolerogenic dendritic cells induce regulatory T cells. Adv Immunol 108:111–165. https://doi.org/10.1016/B978-0-12-380995-7.00004-5
Manuel SL, Rahman S, Wigdahl B, Khan ZK, Jain P (2007) Dendritic cells in autoimmune diseases and neuroinflammatory disorders. Front Biosci 12:4315–4335
Middleton E Jr, Kandaswami C, Theoharides TC (2000) The effects of plant flavonoids on mammalian cells: implications for inflammation, heart disease, and cancer. Pharmacol Rev 52:673–751
Millington C, Sonego S, Karunaweera N, Rangel A, Aldrich-Wright JR, Campbell IL, Gyengesi E, Münch G (2014) Chronic neuroinflammation in Alzheimer's disease: new perspectives on animal models and promising candidate drugs. Biomed Res Int 2014:309129. https://doi.org/10.1155/2014/309129
Mohammad MG et al (2012) Dendritic cells and multiple sclerosis: disease, tolerance and therapy. Int J Mol Sci 14:547–562. https://doi.org/10.3390/ijms14010547
Noubade R et al (2011) Activation of p38 MAPK in CD4 T cells controls IL-17 production and autoimmune encephalomyelitis. Blood 118:3290–3300. https://doi.org/10.1182/blood-2011-02-336552
Noubade R, Milligan G, Zachary JF, Blankenhorn EP, del Rio R, Rincon M, Teuscher C (2007) Histamine receptor H1 is required for TCR-mediated p38 MAPK activation and optimal IFN-gamma production in mice. J Clin Invest 117:3507–3518. https://doi.org/10.1172/JCI32792
Olmos G, Llado J (2014) Tumor necrosis factor alpha: a link between neuroinflammation and excitotoxicity. Mediators Inflamm 2014:861231. https://doi.org/10.1155/2014/861231
Onasanwo SA, Velagapudi R, El-Bakoush A, Olajide OA (2016) Inhibition of neuroinflammation in BV2 microglia by the biflavonoid kolaviron is dependent on the Nrf2/ARE antioxidant protective mechanism. Mol Cell Biochem 414:23–36. https://doi.org/10.1007/s11010-016-2655-8
Ota A, Ulrih NP (2017) An Overview of Herbal Products and Secondary Metabolites Used for Management of Type Two Diabetes. Front Pharmacol 8:436. https://doi.org/10.3389/fphar.2017.00436
Patil RH et al (2016) Anti-inflammatory effect of Apigenin on LPS-induced pro-inflammatory mediators and AP-1 factors in human lung epithelial cells. Inflammation 39:138–147. https://doi.org/10.1007/s10753-015-0232-z
Platzer B, Jorgl A, Taschner S, Hocher B, Strobl H (2004) RelB regulates human dendritic cell subset development by promoting monocyte intermediates. Blood 104:3655–3663. https://doi.org/10.1182/blood-2004-02-0412
Ross JA, Kasum CM (2002) Dietary flavonoids: bioavailability, metabolic effects, and safety. Annual review of nutrition 22:19–34. https://doi.org/10.1146/annurev.nutr.22.111401.144957
Sadraei H, Asghari G, Khanabadi M, Minaiyan M (2017) Anti-inflammatory effect of apigenin and hydroalcoholic extract of Dracocephalum kotschyi on acetic acid-induced colitis in rats. Res Pharm Sci 12:322–329. https://doi.org/10.4103/1735-5362.212050
Sae-Wong C, Matsuda H, Tewtrakul S, Tansakul P, Nakamura S, Nomura Y, Yoshikawa M (2011) Suppressive effects of methoxyflavonoids isolated from Kaempferia parviflora on inducible nitric oxide synthase (iNOS) expression in RAW 264.7 cells. Journal of Ethnopharmacology 136:488–495. https://doi.org/10.1016/j.jep.2011.01.013
Sagar D, Foss C, El Baz R, Pomper MG, Khan ZK, Jain P (2012) Mechanisms of dendritic cell trafficking across the blood-brain barrier. J NeuroImmune Pharmacol 7:74–94. https://doi.org/10.1007/s11481-011-9302-7
Segura E et al (2013) Human inflammatory dendritic cells induce Th17 cell differentiation. Immunity 38:336–348. https://doi.org/10.1016/j.immuni.2012.10.018
Seo HS, Sikder MA, Lee HJ, Ryu J, Lee CJ (2014) Apigenin Inhibits Tumor Necrosis Factor-alpha-Induced Production and Gene Expression of Mucin through Regulating Nuclear Factor-Kappa B Signaling Pathway in Airway Epithelial Cells. Biomol Ther (Seoul) 22:525–531. https://doi.org/10.4062/biomolther.2014.094
Serafini M, Peluso I, Raguzzini A (2010) Flavonoids as anti-inflammatory agents. The Proceedings of the Nutrition Society 69:273–278. https://doi.org/10.1017/S002966511000162X
Shih VF et al (2012) Control of RelB during dendritic cell activation integrates canonical and noncanonical NF-kappaB pathways. Nat Immunol 13:1162–1170. https://doi.org/10.1038/ni.2446
Shukla S, Gupta S (2010) Apigenin: a promising molecule for cancer prevention. Pharm Res 27:962–978. https://doi.org/10.1007/s11095-010-0089-7
Steinman RM (2003) The control of immunity and tolerance by dendritic cell. Pathol Biol (Paris) 51:59–60
Sung B, Chung HY, Kim ND (2016) Role of Apigenin in Cancer prevention via the induction of apoptosis and autophagy. J Cancer Prev 21:216–226. https://doi.org/10.15430/JCP.2016.21.4.216
Thomas R (2013) RelB and the aryl hydrocarbon receptor: dendritic cell tolerance at the epithelial interface. Immunol Cell Biol 91:543–544. https://doi.org/10.1038/icb.2013.51
Tong X, Pelling JC (2013) Targeting the PI3K/Akt/mTOR axis by apigenin for cancer prevention. Anticancer Agents Med Chem 13:971–978
Venigalla M, Gyengesi E, Munch G (2015) Curcumin and Apigenin - novel and promising therapeutics against chronic neuroinflammation in Alzheimer's disease. Neural Regen Res 10:1181–1185. https://doi.org/10.4103/1673-5374.162686
Visavadiya NP et al (2016) Integrin-FAK signaling rapidly and potently promotes mitochondrial function through STAT3 cell. Commun Signal 14:32. https://doi.org/10.1186/s12964-016-0157-7
Wang J, Liu YT, Xiao L, Zhu L, Wang Q, Yan T (2014) Anti-inflammatory effects of apigenin in lipopolysaccharide-induced inflammatory in acute lung injury by suppressing COX-2 and NF-kB pathway. Inflammation 37:2085–2090. https://doi.org/10.1007/s10753-014-9942-x
Wang Q, Cui W, Liu M, Zhang J, Liao R-Q, Liao X-L, Yang J (2015) An improved synthesis of apigenin. Journal of Chemical Research 39:67+
Wang X, Wang W, Wang JZ, Yang C, Liang CZ (2018) Effect of apigenin on apoptosis induced by renal ischemia/reperfusion injury in vivo and in vitro. Ren Fail 40:498–505. https://doi.org/10.1080/0886022X.2018.1497517
Wu GF, Laufer TM (2007) The role of dendritic cells in multiple sclerosis. Curr Neurol Neurosci Rep 7:245–252
Wu Q, Wang Q, Mao G, Dowling CA, Lundy SK, Mao-Draayer Y (2017) Dimethyl Fumarate selectively reduces memory T cells and shifts the balance between Th1/Th17 and Th2 in multiple sclerosis patients. J Immunol 198:3069–3080. https://doi.org/10.4049/jimmunol.1601532
Xie ZX, Zhang HL, Wu XJ, Zhu J, Ma DH, Jin T (2015) Role of the immunogenic and tolerogenic subsets of dendritic cells in multiple sclerosis. Mediators Inflamm 2015:513295. https://doi.org/10.1155/2015/513295
Yan X, Qi M, Li P, Zhan Y, Shao H (2017) Apigenin in cancer therapy: anti-cancer effects and mechanisms of action. Cell Biosci 7:50. https://doi.org/10.1186/s13578-017-0179-x
Yang Y et al (2009) T-bet is essential for encephalitogenicity of both Th1 and Th17 cells. J Exp Med 206:1549–1564. https://doi.org/10.1084/jem.20082584
Yoon MS et al (2006) Apigenin inhibits immunostimulatory function of dendritic cells: implication of immunotherapeutic adjuvant. Mol Pharmacol 70:1033–1044. https://doi.org/10.1124/mol.106.024547
Zanetti M, Castiglioni P, Schoenberger S, Gerloni M (2003) The role of relB in regulating the adaptive immune response. Ann N Y Acad Sci 987:249–257
Zhang X, Wang G, Gurley EC, Zhou H (2014) Flavonoid apigenin inhibits lipopolysaccharide-induced inflammatory response through multiple mechanisms in macrophages. PLoS One 9:e107072. https://doi.org/10.1371/journal.pone.0107072
Zhao L, Wang JL, Wang YR, Fa XZ (2013) Apigenin attenuates copper-mediated beta-amyloid neurotoxicity through antioxidation, mitochondrion protection and MAPK signal inactivation in an AD cell model. Brain Res 1492:33–45. https://doi.org/10.1016/j.brainres.2012.11.019
Availability of Supporting Data
Please contact the corresponding author for requests for obtaining the datasets generated during this study.
Funding
These studies have been funded in part with NIH/NINDS R01 NS097147 to PJ. Authors also wish to acknowledge the National MS Society grant RG 4471A6/2 that supported FB.
Author information
Authors and Affiliations
Contributions
Conception and design (RG, PJ). In vitro DC and T cell assay design and execution (RG, RB, PM). Analysis and interpretation of data (RG). EAE in vivo mouse work (FB) while NS and MN kindly provided with splenocytes from naïve mice. 15-color flow cytometry data acquisition (MB). Drafting the manuscript (RG, PJ). Document revision and editing (RG, RB, PJ, ZKK). Final approval of the version to be published (ZKK, PJ). All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics Approval
The C57BL/6 mice were treated in accordance with NIH guidelines under protocols approved by the Institutional Animal Care and Use Committee of Drexel University.
Consent for Publication
Not applicable.
Competing Interests
The authors declare that they have no competing interests.
Disclosures
The authors declare no financial conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary Figure 1
Antigen independent naïve T cell polarization. Splenocytes were pooled from naïve mice and total T cells were isolated as per the EasySep mouse T cell isolation protocol. T cells were stimulated for 72 h with different mixtures of cytokines and antibodies to induce T cell polarization. The T cells were cultured in the presence or absence of 20 μM Apigenin. T-bet, IL-4, IL-17, and FoxP3 mRNA expression was detected by qPCR and compared to appropriate control (left). Culture supernatants were extracted and cytokines TNF-α, IL-17, IL-4, and TGF-β levels were detected by ELISA (right). Each data point is representative of 2 individual experiments with two replicates (PDF 119 kb)
Supplementary Figure 2
Reduced RelB expression in EAE and naïve mouse splenocytes. Splenocytes from EAE and naïve mice were stimulated with and without MOG35–55 peptide for 3 days in the presence or absence of 20 μM Apigenin. Cells were pelleted, and RNA was extracted using the TRIzol method followed by cDNA preparation and qPCR. A) RelB mRNA expression compared to β-Actin and normalized to cells that were not treated with MOG35–55 peptide. Naïve T cells that were polarized as described in Fig. 5 were analyzed for RelB mRNA expression in the Th1, Th2 and Th17 subsets. B) RelB mRNA expression compared to β-Actin and normalized to the Th0 subset. Sample standard error (SE) is shown and statistical significance was determined by Student’s t test. (**p < 0.01). (PDF 41 kb)
Supplementary Figure 3
RelB silencing does not potentiate Apigenin effect in immature DCs. DCs were isolated as in Fig. 1 followed by treatment with 20 μM Apigenin. 25 nM siRNA directed against RelB was transfected in to DCs. sip65 was used as positive control. Changes in DC phenotype were determined by flow cytometry. Figures show FACS plot of cell surface markers expressed on DCs. (PDF 309 kb)
Supplementary Figure 4
RelB silencing does not significantly modulate differentiation of Th2 cells. DCs and autologous PBLs from 3 donors were treated as described in Fig. 8. Enumeration of the Th2 cell subset upon both RelB knockdown and Apigenin treatment is shown. Statistical significance was determined by 2-way ANOVA using Sidak’s test for multiple comparisons. (PDF 44 kb)
Supplementary Figure 5
RelB silencing increases numbers of Tregs cultured in the presence of Apigenin treated immature DCs. DCs were isolated as described. DCs were treated with 20 μM Apigenin or left untreated. 25 nM siRelB was transfected in to DCs. sip65 was used as positive control. Following transfection DCs were co-cultured with autologous PBLs for 6d as described. FACS plots describe T cell subset polarization in presence of immature DCs treated/untreated with Apigenin. (PDF 977 kb) (PDF 465 kb)
Rights and permissions
About this article
Cite this article
Ginwala, R., Bhavsar, R., Moore, P. et al. Apigenin Modulates Dendritic Cell Activities and Curbs Inflammation Via RelB Inhibition in the Context of Neuroinflammatory Diseases. J Neuroimmune Pharmacol 16, 403–424 (2021). https://doi.org/10.1007/s11481-020-09933-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11481-020-09933-8