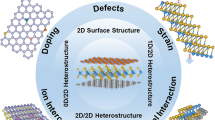
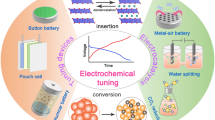
Abstract
Electrocatalytic materials are a critical bottleneck for the development of new energy economics. This review summarizes the unique physicochemical properties of topological, magnetic, and rare earth materials and their applications in the functionalization of electrocatalysts. Topological materials have unique band structures and geometric structures, and the interface difference in charge transport structures can give rise to topological insulators, topological superconductors, and Dirac metals. Magnetic materials possess distinctive electron spin-splitting configurations, and varying spin strengths induce disparate impacts on the intermediate equilibrium adsorption capability. Rare earth materials have unique f-electron roaming properties, broad atomic radius, and f-orbital configurations, which typically confer notable advantages in oxygen reduction reactions. Furthermore, the catalytic performance exhibits significant differences under an external alternating electric, thermal, and magnetic field. These new materials show great potential in the re-functionalization of electrocatalytic materials and are expected to lead the development of the next generation of emerging energy materials.
Similar content being viewed by others
References
Shi G Q, Xue D F. Perspective on multiple degrees of freedom in crystal materials. Sci China Tech Sci, 2022, 65: 2787–2789
He T, Wang W, Shi F, et al. Mastering the surface strain of platinum catalysts for efficient electrocatalysis. Nature, 2021, 598: 76–81
Li C T, Tsai Y L, Ho K C. Earth abundant silicon composites as the electrocatalytic counter electrodes for dye-sensitized solar cells. ACS Appl Mater Interfaces, 2016, 8: 7037–7046
Xu W, Wang G, Liu Q, et al. Doping vacancy synergy engineering: Ce-doped FeNi-Sx micro-succulent ameliorating electrocatalytic oxygen evolution performance. Electrochim Acta, 2022, 431: 141133
Chen L, Guo M, Zhang H F, et al. Characterization and electrocatalytic properties of PtRu/C catalysts prepared by impregnationreduction method using Nd2O3 as dispersing reagent. Electrochim Acta, 2006, 52: 1191–1198
Gao W, Ma F, Wang C, et al. Ce dopant significantly promotes the catalytic activity of Ni foam-supported Ni3S2 electrocatalyst for alkaline oxygen evolution reaction. J Power Sources, 2020, 450: 227654
Wang W, Li D, Cui T. Carbon and oxygen coordinating atoms adjust transition metal single-atom catalysts based on boron nitride monolayers for highly efficient CO2 electroreduction. ACS Appl Mater Interfaces, 2021, 13: 18934–18943
Seh Z W, Kibsgaard J, Dickens C F, et al. Combining theory and experiment in electrocatalysis: Insights into materials design. Science, 2017, 355: eaad4998
Bao C, Tang P, Sun D, et al. Light-induced emergent phenomena in 2D materials and topological materials. Nat Rev Phys, 2021, 4: 33–48
Qin W, Li L, Zhang Z. Chiral topological superconductivity arising from the interplay of geometric phase and electron correlation. Nat Phys, 2019, 15: 796–802
Wang Y, Wang B, Jiang W, et al. Sub-2 nm ultra-thin Bi2O2CO3 nanosheets with abundant Bi-O structures toward formic acid electrosynthesis over a wide potential window. Nano Res, 2021, 15: 2919–2927
Su J, Pan D, Dong Y, et al. Ultrafine Fe2C iron carbide nanoclusters trapped in topological carbon defects for efficient electroreduction of carbon dioxide. Adv Energy Mater, 2023, 13: 2204391
Liu D, Lu M, Liu D, et al. Heat-triggered ferri-to-paramagnetic transition accelerates redox couple-mediated electrocatalytic water oxidation. Adv Funct Mater, 2022, 32: 2111234
Ma Y, Wang Y, Cong J, et al. Magnetic-field tuning of hydrogen bond order-disorder transition in metal-organic frameworks. Phys Rev Lett, 2019, 122: 255701
Shen B, Zhang Y, Komijani Y, et al. Strange-metal behaviour in a pure ferromagnetic Kondo lattice. Nature, 2020, 579: 51–55
Roy C, Knudsen B P, Pedersen C M, et al. Scalable synthesis of carbon-supported platinum-lanthanide and rare-earth alloys for oxygen reduction. ACS Catal, 2018, 8: 2071–2080
Wang X, Wang J, Wang P, et al. Engineering 3d-2p-4f gradient orbital coupling to enhance electrocatalytic oxygen reduction. Adv Mater, 2022, 34: e2206540
Bai Y L, Xue M M, Zhao Z Q, et al. Functionalizations of boron nitride nanostructures. Sci China Tech Sci, 2021, 64: 1–10
Jiang S, Li Z, Wang H, et al. Tuning nondoped carbon nanotubes to an efficient metal-free electrocatalyst for oxygen reduction reaction by localizing the orbital of the nanotubes with topological defects. Nanoscale, 2014, 6: 14262–14269
Chen C, You J, Feng H J, et al. A multi-objective study on the constructal design of non-uniform heat generating disc cooled by radialand dendritic-pattern cooling channels. Sci China Tech Sci, 2021, 64: 729–744
Chen G, Lin X, Liu Y, et al. Monolayer MoS2-based transistors with low contact resistance by inserting ultrathin Al2O3 interfacial layer. Sci China Tech Sci, 2023, 66: 1831–1840
He Y, Yan D, Wang S, et al. Topological type-II dirac semimetal and superconductor PdTe2 for ethanol electrooxidation. Energy Technol, 2019, 7: 1900663
Rinaldi G, Nakajo A, Burdet P, et al. Characterization of local morphology and availability of triple-phase boundaries in solid oxide cell electrodes. Acta Mater, 2019, 178: 194–206
Li G, Sun Y, Rao J, et al. Carbon-tailored semimetal MoP as an efficient hydrogen evolution electrocatalyst in both alkaline and acid media. Adv Energy Mater, 2018, 8: 1801258
Huang Y, Wang Y, Tang C, et al. Atomic modulation and structure design of carbons for bifunctional electrocatalysis in metal-air batteries. Adv Mater, 2019, 31: 1803800
Katzbear R R, Zhu Y, Mao Z, et al. Persistence and evolution of materials features during catalysis using topological and trivial polymorphs of MoTe2. ChemCatChem, 2022, 14: e202101714
Peng X, Meng L, Zhang W, et al. Facile preparation of nitrogen-doped graphene scrolls via acoustic cavitation as electrocatalyst for glucose biosensing. J Solid State Electrochem, 2015, 20: 439–447
Qu Q, Liu B, Liang J, et al. Expediting hydrogen evolution through topological surface states on Bi2Te3. ACS Catal, 2020, 10: 2656–2666
Tandon S, Steuber F W, Kathalikkattil A C, et al. Modulating structural and electronic properties of rare archimedean and Johnson-type Mn cages. Inorg Chem, 2021, 60: 8388–8393
Zhao Y, Tang H, Yang N, et al. Graphdiyne: Recent achievements in photo- and electrochemical conversion. Adv Sci, 2018, 5: 1800959
Ye R, Tong Y, Feng D, et al. A topological chemical transition strategy of bismuth-based materials for high-efficiency electrocatalytic carbon dioxide conversion to formate. J Mater Chem A, 2023, 11: 4691–4702
Liu M, Spanos P D, Yu S H. Synthesis of ultrathin Bi2Se3 nanosheets/graphene nanocomposite with defects/vacancies-dependent transient photocurrent performance. Nano Energy, 2019, 64: 103877
Wang A, Peng J, Ren N, et al. Serendipity for topological insulator as multifunctional electrocatalyst. ACS Appl Energy Mater, 2020, 3: 8929–8936
Zhao Y, Zhang L, Xiang J, et al. Topological tailoring-induced Dirac cone in ultrathin niobium diboride nanosheets for electrocatalytic sulfur reduction reaction. Mater Today Phys, 2023, 32: 101029
Du Y P, Jin X N, Han H G, et al. Reusable electronic products value prediction based on reinforcement learning. Sci China Tech Sci, 2022, 65: 1578–1586
Jiang C J, Guo R. Liquid metal-based paper electronics: Materials, methods, and applications. Sci China Tech Sci, 2023, 66: 1595–1616
Dong Y, Zhang Q, Tian Z, et al. Ammonia thermal treatment toward topological defects in porous carbon for enhanced carbon dioxide electroreduction. Adv Mater, 2020, 32: 2001300
Jia Y, Chen J, Yao X. Defect electrocatalytic mechanism: Concept, topological structure and perspective. Mater Chem Front, 2018, 2: 1250–1268
Liu L, Lu J Y, Long X L, et al. 3D printing of high-performance micro-supercapacitors with patterned exfoliated graphene/carbon nanotube/ silver nanowire electrodes. Sci China Tech Sci, 2021, 64: 1065–1073
Ye Y K, Weng M Y, Zhang W T, et al. Calculating electron-phonon coupling matrix: Theory introduction, code development and preliminary application. Sci China Tech Sci, 2022, 66: 204–214
Zhao L W, Gu T T, Liang Z W, et al. Recent advances in bifunctional catalysts for zinc-air batteries: Synthesis and potential mechanisms. Sci China Tech Sci, 2022, 65: 2221–2245
Jiang P, Jiang K, Tranca D, et al. Rational control of topological defects in porous carbon for high-efficiency carbon dioxide conversion. Adv Mater Interfaces, 2021, 8: 2100051
Tang C, Wang H F, Chen X, et al. Topological defects in metal-free nanocarbon for oxygen electrocatalysis. Adv Mater, 2016, 28: 6845–6851
Ye G, Liu S, Zhao K, et al. Singlet oxygen induced site-specific etching boosts nitrogen-carbon sites for high-efficiency oxygen reduction. Angew Chem Int Ed, 2023, 62: e202303409
Fan M P, Chen Y M, Ke X, et al. In situ growth of NiS2 nanosheet array on Ni foil as cathode to improve the performance of lithium/ sodium-sulfur batteries. Sci China Tech Sci, 2022, 65: 231–237
Fang C, An W. Single-metal-atom site with high-spin state embedded in defective BN nanosheet promotes electrocatalytic nitrogen reduction. Nano Res, 2021, 14: 4211–4219
Gunasekara T, Tong Y, Speelman A L, et al. Role of high-spin species and pendant amines in electrocatalytic alcohol oxidation by a nickel phosphine complex. ACS Catal, 2022, 12: 2729–2740
Mei Z Y, Zhao G, Xia C, et al. Regulated high-spin state and constrained charge behavior of active cobalt sites in covalent organic frameworks for promoting electrocatalytic oxygen reduction. Angew Chem Int Ed, 2023, 62: e202303871
Do V H, Lee J M. Orbital occupancy and spin polarization: From mechanistic study to rational design of transition metal-based electrocatalysts toward energy applications. ACS Nano, 2022, 16: 17847–17890
Yang H, He Q, Liu Y, et al. On-chip electrocatalytic microdevice: An emerging platform for expanding the insight into electrochemical processes. Chem Soc Rev, 2020, 49: 2916–2936
Chen S, Gao Y, Wang W, et al. Prediction of three-metal cluster catalysts on two-dimensional W2N3 support with integrated descriptors for electrocat-alytic nitrogen reduction. ACS Nano, 2023, 17: 1522–1532
Kwon I S, Kwak I H, Zewdie G M, et al. MoSe2-VSe2-NbSe2 ternary alloy nanosheets to boost electrocatalytic hydrogen evolution reaction. Adv Mater, 2022, 34: e2205524
Dai Y, Liu B, Zhang Z, et al. Tailoring the d-orbital splitting manner of single atomic sites for enhanced oxygen reduction. Adv Mater, 2023, 35: e2210757
Yuan M, Chen J, Zhang H, et al. Host-guest molecular interaction promoted urea electrosynthesis over a precisely designed conductive metal-organic framework. Energy Environ Sci, 2022, 15: 2084–2095
Li N, Wang W, Zhu L, et al. A novel electro-cleanable PAN-ZnO nanofiber membrane with superior water flux and electrocatalytic properties for organic pollutant degradation. Chem Eng J, 2021, 421: 127857
Kwak I H, Kwon I S, Kim J Y, et al. Full composition tuning of W1-xNbxSe2 alloy nanosheets to promote the electrocatalytic hydrogen evolution reaction. ACS Nano, 2022, 16: 13949–13958
Ren L, Wen X, Du D, et al. Engineering high-spin state cobalt cations in spinel ZnCo2O4 for spin channel propagation and electrocatalytic activity enhancement in Li-O2 battery. Chem Eng J, 2023, 462: 142288
Gong X, Jiang Z, Zeng W, et al. Alternating magnetic field induced magnetic heating in ferromagnetic cobalt single-atom catalysts for efficient oxygen evolution reaction. Nano Lett, 2022, 22: 9411–9417
Zheng F, Zhang W, Zhang X, et al. Sub-2 nm ultrathin and robust 2D FeNi layered double hydroxide nanosheets packed with 1D FeNi- MOFs for enhanced oxygen evolution electrocatalysis. Adv Funct Mater, 2021, 31: 2103318
Sheng Q L, Yu H, Zheng J B. Sol-gel derived carbon ceramic electrode for the investigation of the electrochemical behavior and electrocatalytic activity of neodymium hexacyanoferrate. Electrochim Acta, 2007, 52: 4506–4512
Xiao N, Qiao Y Q, Shi N K, et al. Realization of high thermal conductivity and tunable thermal expansion in the ScF3@Cu coreshell composites. Sci China Tech Sci, 2021, 64: 2057–2065
Xu G P, Zheng Y B, Feng Y G, et al. A triboelectric/electromagnetic hybrid generator for efficient wind energy collection and power supply for electronic devices. Sci China Tech Sci, 2021, 64: 2003–2011
Zhang X, Deng Z S. Roadmap towards new generation liquid metal thermal interface materials. Sci China Tech Sci, 2023, 66: 1530–1550
Li D, Sun L, Hu L, et al. Rare earth in situ-doped ZIF-67 derived N doped C encapsulated Sm2O3/Co nanoparticles as excellent oxygen reduction reaction catalyst for Al-air batteries. J Power Sources, 2021, 482: 229052
Wu Z, Wang Z, Geng F. Radially aligned hierarchical nickel/nickel-iron (oxy)hydroxide nanotubes for efficient electrocatalytic water splitting. ACS Appl Mater Interfaces, 2018, 10: 8585–8593
Mutharani B, Keerthi M, Chen S M, et al. One-pot sustainable synthesis of Ce2S3/gum arabic carbon flower nanocomposites for the detection of insecticide imidacloprid. ACS Appl Mater Interfaces, 2020, 12: 4980–4988
Rosalbino F, Borzone G, Angelini E, et al. Hydrogen evolution reaction on NiRE (RE=rare earth) crystalline alloys. Electrochim Acta, 2003, 48: 3939–3944
Santos D M F, Šljukić B, Amaral L, et al. Nickel-rare earth electrodes for sodium borohydride electrooxidation. Electrochim Acta, 2016, 190: 1050–1056
Smith A J, Chang Y H, Raidongia K, et al. Molybdenum sulfide supported on crumpled graphene balls for electrocatalytic hydrogen production. Adv Energy Mater, 2014, 4: 1400398
Gong Y N, Cao C Y, Shi W J, et al. Modulating the electronic structures of dual-atom catalysts via coordination environment engineering for boosting CO2 electroreduction. Angew Chem Int Ed, 2022, 61: e202215187
Gao H M, Xiao M L, Li G Q, et al. Oxygen-vacancy-rich TiO2 enables highly active and durable water electrolysis of urchin-like RuO2 catalyst. Sci China Tech Sci, 2022, 65: 2317–2324
Qu C C, Cao L X, Li M L, et al. Liquid metal-based plant electronic tattoos for in-situ monitoring of plant physiology. Sci China Tech Sci, 2023, 66: 1617–1628
Wang X P, Guo J R, Hu L. Preparation and application of gallium-based conductive materials in the very recent years. Sci China Tech Sci, 2020, 64: 681–695
An X S, Fan Y J, Chen D J, et al. Enhanced activity of rare earth doped PtRu/C catalysts for methanol electro-oxidation. Electrochim Acta, 2011, 56: 8912–8918
Xiang S, Wang L, Huang C C, et al. Concave cubic PtLa alloy nanocrystals with high-index facets: Controllable synthesis in deep eutectic solvents and their superior electrocatalytic properties for ethanol oxidation. J Power Sources, 2018, 399: 422–428
Chen Z, Yang H, Mebs S, et al. Reviving oxygen evolution electro-catalysis of bulk La-Ni intermetallics via gaseous hydrogen engineering. Adv Mater, 2023, 35: e2208337
Wang W, Xue S, Li J, et al. Cerium carbide embedded in nitrogen-doped carbon as a highly active electrocatalyst for oxygen reduction reaction. J Power Sources, 2017, 359: 487–493
Chhetri M, Rana M, Loukya B, et al. Mechanochemical synthesis of free-standing platinum nanosheets and their electrocatalytic properties. Adv Mater, 2015, 27: 4430–4437
Xu M, Wang Z, Wang F, et al. Fabrication of cerium doped Ti/nanoTiO2/PbO2 electrode with improved electrocatalytic activity and its application in organic degradation. Electrochim Acta, 2016, 201: 240–250
Zhang X, Cui T, Zhao X, et al. Electrochemical difunctionalization of alkenes by a four-component reaction cascade mumm rearrangement: Rapid access to functionalized imides. Angew Chem Int Ed, 2020, 59: 3465–3469
Zhou J, He J, Wang T, et al. Synergistic effect of RE2O3 (RE=Sm, Eu and Gd) on Pt/mesoporous carbon catalyst for methanol electro-oxidation. Electrochim Acta, 2009, 54: 3103–3108
Liu Y, Liu H, Ma J, et al. Investigation on electrochemical properties of cerium doped lead dioxide anode and application for elimination of nitrophenol. Electrochim Acta, 2011, 56: 1352–1360
Yuan X, Li J, Zhang C, et al. Fabrication of Pt3Ni catalysts on polypyrrole- modified electrochemically exfoliated graphene with exceptional electrocatalytic performance for methanol and ethanol oxidation. Electrochim Acta, 2020, 340: 135969
Zhou Y, Li Z, Hao C, et al. Electrocatalysis enhancement of α,β-PbO2 nanocrystals induced via rare earth Er(III) doping strategy: Principle, degradation application and electrocatalytic mechanism. Electrochim Acta, 2020, 333: 135535
Sakthivel M, Sukanya R, Chen S M, et al. Synthesis and characterization of samarium-substituted molybdenum diselenide and its graphene oxide nanohybrid for enhancing the selective sensing of chloramphenicol in a milk sample. ACS Appl Mater Interfaces, 2018, 10: 29712–29723
Abbott D F, Pittkowski R K, Macounova K, et al. Design and synthesis of Ir/Ru pyrochlore catalysts for the oxygen evolution reaction based on their bulk thermodynamic properties. ACS Appl Mater Interfaces, 2019, 11: 37748–37760
Li N, Xu X, Luo D, et al. Electrocatalytic activities of REMn2O5 (RE=Dy, Ho, Er, Tm, Yb, and Lu) and Er0.76Zr0.11Ca0.13Mn2O5 for oxygen reduction in alkaline solution. J Power Sources, 2004, 126: 229–235
Fan C, Wang X, Wu X, et al. Neodymium-evoked valence electronic modulation to balance reversible oxygen electrocatalysis. Adv Energy Mater, 2022, 13: 2203244
Karfa P, Majhi K C, Madhuri R. Shape-dependent electrocatalytic activity of iridium oxide decorated erbium pyrosilicate toward the hydrogen evolution reaction over the entire pH range. ACS Catal, 2018, 8: 8830–8843
Zhang W, Zhang L, Guan K, et al. Effective promotion of oxygen reduction activity by rare earth doping in simple perovskite cathodes for intermediate-temperature solid oxide fuel cells. J Power Sources, 2020, 446: 227360
Zhang X, Yamauchi K, Sakai K. Earth-abundant photocatalytic CO2 reduction by multielectron chargeable cobalt porphyrin catalysts: High CO/H2 selectivity in water based on phase mismatch in frontier MO association. ACS Catal, 2021, 11: 10436–10449
Author information
Authors and Affiliations
Corresponding author
Additional information
This work was supported by the National Natural Science Foundation of China (Grant Nos. 52203303, 52220105010, M-0755), the Natural Science Foundation of Guangdong Province (Grant No. 2022A1515010076), the Natural Science Foundation of Shandong Province (Grant No. ZR2020ZD35), the SIAT Innovation Program for Excellent Young Researchers (Grant No. E2G017), and the CAS president’s international fellowship initiative grant (Grant Nos. 2022VEA0011, 2022VEA0016, 2022VEA0017), as well as the Shenzhen Science and Technology Program (Grant No. SGDX20211123151002003).
Supporting Information
The supporting information is available online at tech.scichina.com and link.springer.com. The supporting materials are published as submitted, without typesetting or editing. The responsibility for scientific accuracy and content remains entirely with the authors.
Supporting Information
Rights and permissions
About this article
Cite this article
Zhang, D., Peng, C. & Xue, D. Design considerations for re-functionalizing electrocatalytic materials. Sci. China Technol. Sci. 66, 3355–3368 (2023). https://doi.org/10.1007/s11431-023-2454-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11431-023-2454-9