Abstract
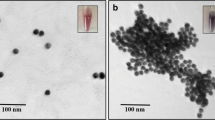
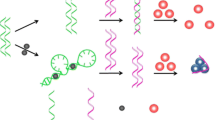
Organophosphorous pesticide (OP) contamination could have serious adverse effects on human health and the environment. Due to the toxicity of OPs and the threat presented by their accidental or intentional release in populated areas, the determination and monitoring of these OPs in food products and environment is of great importance. OPs are present in very small quantities and therefore, methods for their detection need to be highly sensitive and selective. Here, we aimed to develop a simple and selective aptamer-based colorimetric assay for the detection of omethoate, which is one of the commonly used OPs. The principle of the assay is that single-stranded DNA (ssDNA)-wrapped gold nanoparticles (AuNPs) are resistant to salt-induced aggregation. By employing an “artificial antibody” organophosphorous pesticide-binding aptamer (OBA) as the recognition element, aptamer-wrapped AuNPs (Au-apta) show high selectivity towards omethoate, resulting in the disconnection of aptamers from AuNPs and the aggregation of AuNPs. As there is a significant color change from the interparticle plasmon coupling during the aggregation of AuNPs, the established assay showed good linearity between 0.1 and 10 μmol/L, with a low detection limit of 0.1 μmol/L. Other OPs such as profenofos, phorate, and isocarbophos would not interfere with the detection of omethoate despite having similar structures. Thus, the colorimetric method shows potential for use in the detection of omethoate in real soil samples.
Similar content being viewed by others
References
Darko G, Acquaah SO. Levels of organochlorine pesticides residues in meat. Int J Environ Sci Technol, 2007, 4: 521–524
Chen XY, Panuwet P, Hunter RE, Riederer AM, Bernoudy GC, Barr DB, Ryan PB. Method for the quantification of current use and persistent pesticides in cow milk, human milk and baby formula using gas chromatography tandem mass spectrometry. J Chromatogr B, 2014, 970: 121–130
Fillion J, Sauve F, Selwyn J. Multiresidue method for the determination of residues of 251 pesticides in fruits and vegetables by gas chromatography/mass spectrometry and liquid chromatography with fluorescence detection. J AOAC Int, 2000, 83: 698–713
Goldman LR, Koduru S. Chemicals in the environmental and developmental toxicity to children: a public health and policy perspective. Environ Health Persp, 2000, 108: 443–448
Graslund S, Bengtsson BE. Chemicals and biological products used in south-east asian shrimp farming, and their potential impact on the environment—a review. Sci Total Environ, 2001, 280: 93–131
Simcox NJ, Fenske RA, Wolz SA, Lee IC, Kalman DA. Pesticides in household dust and soil: exposure pathways for children of agricultural families. Environ Health Persp, 1995, 103: 1126–1134
Kutz FW, Wood PH, Bottimore DP. Organochlorine pesticides and polychlorinated biphenyls in human adipose tissue. In: Ware GW, Ed. Reviews of Environmental Contamination and Toxicology. Volume 120. New York: Springer, 1991. 1–82
Farahat TM, Abdelrasoul GM, Amr MM, Shebl MM, Farahat FM, Anger WK. Neurobehavioural effects among workers occupationally exposed to organophosphorous pesticides. Occup Environ Med, 2003, 60: 279–286
Fulton MH, Key PB. Acetylcholinesterase inhibition in estuarine fish and invertebrates as an indicator of organophosphorus insecticide exposure and effects. Environ Toxicol Chem, 2001, 20: 37–45
Sanchez-Pena LC, Reyes BE, Lopez-Carrillo L, Recio R, Moran-Martinez J, Cebrian ME, Quintanilla-Vega B. Organophosphorous pesticide exposure alters sperm chromatin structure in mexican agricultural workers. Toxicol Appl Pharm, 2004, 196: 108–113
Worek F, Thiermann H, Szinicz L, Eyer P. Kinetic analysis of interactions between human acetylcholinesterase, structurally different organophosphorus compounds and oximes. Biochem Pharm, 2004, 68: 2237–2248
Rodriguez-Mozaz S, de Alda MJL, Barceló D. Monitoring of estrogens, pesticides and bisphenol a in natural waters and drinking water treatment plants by solid-phase extraction-liquid chromatographymass spectrometry. J Chromatogr A, 2004, 1045: 85–92
Nagaraju D, Huang SD. Determination of triazine herbicides in aqueous samples by dispersive liquid-liquid microextraction with gas chromatography-ion trap mass spectrometry. J Chromatogr A, 2007, 1161: 89–97
Ferrer I, Thurman EM. Multi-residue method for the analysis of 101 pesticides and their degradates in food and water samples by liquid chromatography/time-of-flight mass spectrometry. J Chromatogr A, 2007, 1175: 24–37
Wu LJ, Song Y, Hu MZ, Zhang HQ, Yu AM, Yu C, Ma Q, Wang ZM. Application of magnetic solvent bar liquid-phase microextraction for determination of organophosphorus pesticides in fruit juice samples by gas chromatography mass spectrometry. Food Chem, 2015, 176: 197–204
Rodriguez-Mozaz S, de Alda MJL, Barceló D. Advantages and limitations of on-line solid phase extraction coupled to liquid chromatography- mass spectrometry technologies versus biosensors for monitoring of emerging contaminants in water. J Chromatogr A, 2007, 1152: 97–115
Alvarez M, Calle A, Tamayo J, Lechuga LM, Abad A, Montoya A. Development of nanomechanical biosensors for detection of the pesticide DDT. Biosens Bioelectron, 2003, 18: 649–653
Andreescu S, Marty JL. Twenty years research in cholinesterase biosensors: from basic research to practical applications. Biomol Eng, 2006, 23: 1–15
Mulchandani A, Chen W, Mulchandani P, Wang J, Rogers KR. Biosensors for direct determination of organophosphate pesticides. Biosens Bioelectron, 2001, 16: 225–230
Bachmann TT, Leca B, Vilatte F, Marty JL, Fournier D, Schmid RD. Improved multianalyte detection of organophosphates and carbamates with disposable multielectrode biosensors using recombinant mutants of drosophila acetylcholinesterase and artificial neural networks. Biosens Bioelectron, 2000, 15: 193–201
Du D, Chen S, Cai J, Zhang A. Immobilization of acetylcholinesterase on gold nanoparticles embedded in sol-gel film for amperometric detection of organophosphorous insecticide. Biosens Bioelectron, 2007, 23: 130–134
Liu GD, Lin YH. Biosensor based on self-assembling acetylcholinesterase on carbon nanotubes for flow injection/amperometric detection of organophosphate pesticides and nerve agents. Anal Chem, 2006, 78: 835–843
Sun X, Wang X. Acetylcholinesterase biosensor based on prussian blue-modified electrode for detecting organophosphorous pesticides. Biosens Bioelectron, 2010, 25: 2611–2614
Viswanathan S, Radecka H, Radecki J. Electrochemical biosensor for pesticides based on acetylcholinesterase immobilized on polyaniline deposited on vertically assembled carbon nanotubes wrapped with ssdna. Biosens Bioelectron, 2009, 24: 2772–2777
Ellington AD, Szostak JW. In vitro selection of RNA molecules that bind specific ligands. Nature, 1990, 346: 818–822
Song S, Wang L, Li J, Fan C, Zhao J. Aptamer-based biosensors. TrAC-Trend Anal Chem, 2008, 27: 108–117
Liu J, Lu Y. Fast colorimetric sensing of adenosine and cocaine based on a general sensor design involving aptamers and nanoparticles. Angew Chem Int Ed, 2006, 118: 96–100
Stojanovic MN, de Prada P, Landry DW. Fluorescent sensors based on aptamer self-assembly. J Am Chem Soc, 2000, 122: 11547–11548
Yao CY, Qi YZ, Zhao YH, Xiang Y, Chen QH, Fu WL. Aptamerbased piezoelectric quartz crystal microbalance biosensor array for the quantification of IgE. Biosens Bioelectron, 2009, 24: 2499–2503
He P, Liu LJ, Qiao WP, Zhang SS. Ultrasensitive detection of thrombin using surface plasmon resonance and quartz crystal microbalance sensors by aptamer-based rolling circle amplification and nanoparticle signal enhancement. Chem Commun, 2014, 50: 1481–1484
Zuo XL, Song SP, Zhang J, Pan D, Wang LH, Fan C. A targetresponsive electrochemical aptamer switch (treas) for reagentless detection of nanomolar ATP. J Am Chem Soc, 2007, 129: 1042–1043
Moreau J, Challier L, Lalaoui N, Mavré F, Noël V, Limoges B, Schöllhorn B, Fave C. Rational design of a redox-labeled chiral target for an enantioselective aptamer-based electrochemical binding assay. Chem Eur J, 2014, 20: 2953–2959
Taleat Z, Cristea C, Marrazza G, Mazloum-Ardakani M, Sandulescu R. Electrochemical immunoassay based on aptamer-protein interaction and functionalized polymer for cancer biomarker detection. J Electroanal Chem, 2014, 717: 119–124
Orendorff CJ, Sau TK, Murphy CJ. Shape-dependent plasmonresonant gold nanoparticles. Small, 2006, 2: 636–639
Wilson R. The use of gold nanoparticles in diagnostics and detection. Chem Soc Rev, 2008, 37: 2028–2045
Wang ZX, Ma LN. Gold nanoparticle probes. Coordin Chem Rev, 2009, 253: 1607–1618
Popovtzer R. Biomedical applications of gold nanomaterials. Nanomedicine, 2014, 9: 1903–1904
Li HX, Rothberg L. Colorimetric detection of DNA sequences based on electrostatic interactions with unmodified gold nanoparticles. Proc Natl Acad Sci USA, 2004, 101: 14036–14039
Zhang J, Wang LH, Pan D, Song SP, Boey FYC, Zhang H, Fan C. Visual cocaine detection with gold nanoparticles and rationally engineered aptamer structures. Small, 2008, 4: 1196–1200
Guarise C, Pasquato L, De Filippis V, Scrimin P. Gold nanoparticlesbased protease assay. Proc Natl Acad Sci USA, 2006, 103: 3978–3982
Peng MP, Ma W, Long YT. Alcohol dehydrogenase-catalyzed gold nanoparticle seed-mediated growth allows reliable detection of disease biomarkers with the naked eye. Anal Chem, 2015, 87: 5891–5896
Wang L, Liu XJ, Zhang Q, Zhang CZ, Liu Y, Tu K, Tu J. Selection of DNA aptamers that bind to four organophosphorus pesticides. Biotechnol Lett, 2012, 34: 869–874
Oujji NB, Bakas I, Istamboulie G, Ait-Ichou I, Ait-Addi E, Rouillon R, Noguer T. A simple colorimetric enzymatic-assay, based on immobilization of acetylcholinesterase by adsorption, for sensitive detection of organophosphorus insecticides in olive oil. Food Control, 2014, 46: 75–80
Han Z, Chi CS, Bai, B, Liu G, Rao QX, Peng SJ, Liu H, Zhao ZH, Zhang DB, Wu AB. Chromogenic platform based on recombinant drosophila melanogaster acetylcholinesterase for visible unidirectional assay of organophosphate and carbamate insecticide residues. Anal Chim Acta, 2012, 720: 126–133
Guo XS, Zhang XY, Cai Q, Shen T, Zhu SM. Developing a novel sensitive visual screening card for rapid detection of pesticide residues in food. Food Control, 2013, 30: 15–23
Pang S, Labuza TP, He L. Development of a single aptamer-based surface enhanced raman scattering method for rapid detection of multiple pesticides. Analyst, 2014, 139: 1895–1901
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Wang, P., Wan, Y., Ali, A. et al. Aptamer-wrapped gold nanoparticles for the colorimetric detection of omethoate. Sci. China Chem. 59, 237–242 (2016). https://doi.org/10.1007/s11426-015-5488-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11426-015-5488-5