Abstract
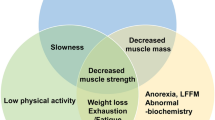
The escalation of life expectancy is accompanied by an increase in the prevalence of age-related conditions, such as sarcopenia. Sarcopenia, a muscle condition defined by low muscle strength, muscle quality or quantity, and physical performance, has a high prevalence among the elderly and is associated to increased mortality. The neuromuscular system has been emerging as a key contributor to sarcopenia pathogenesis. Indeed, the age-related degeneration of the neuromuscular junction (NMJ) function and structure may contribute to the loss of muscle strength and ultimately to the loss of muscle mass that characterize sarcopenia. The present mini-review discusses important signaling pathways involved in the function and maintenance of the NMJ, giving emphasis to the ones that might contribute to sarcopenia pathogenesis. Some conceivable biomarkers, such as C-terminal agrin fragment (CAF) and brain-derived neurotrophic factor (BDNF), and therapeutic targets, namely acetylcholine and calcitonin gene–related peptide (CGRP), can be retrieved, making way to future studies to validate their clinical use.

Similar content being viewed by others
References
World Health Organization. Decade of healthy ageing: baseline report. Summary. Geneva: World Health Organization; 2021.
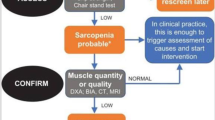
Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyère O, Cederholm T, et al. Sarcopenia: revised european consensus on definition and diagnosis. Age Ageing. 2019;48:16–31. https://doi.org/10.1093/ageing/afy169.
Rossi AP, Rubele S, D’Introno A, Zoico E, Brandimarte P, Amadio G, et al. An update on methods for sarcopenia diagnosis: from bench to bedside. Ital J Med. 2018;12:97–107. https://doi.org/10.4081/itjm.2018.995.
Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, et al. Sarcopenia: european consensus on definition and diagnosis. Age Ageing. 2010;39:412–23. https://doi.org/10.1093/ageing/afq034.
Yazar T, Olgun YH. Prevalance of sarcopenia according to decade. Clin Nutr ESPEN. 2019;29:137–41. https://doi.org/10.1016/j.clnesp.2018.11.005.
Beaudart C, Zaaria M, Pasleau F, Reginster J-Y, Bruyère O. Health outcomes of sarcopenia: a systematic review and meta-analysis. PLoS ONE. 2017;12:e0169548. https://doi.org/10.1371/journal.pone.0169548.
Wiedmer P, Jung T, Castro JP, Pomatto LCD, Sun PY, Davies KJA, et al. Sarcopenia – molecular mechanisms and open questions. Ageing Res Rev. 2021;65:101200. https://doi.org/10.1016/j.arr.2020.101200.
Ibebunjo C, Chick JM, Kendall T, Eash JK, Li C, Zhang Y, et al. Genomic and proteomic profiling reveals reduced mitochondrial function and disruption of the neuromuscular junction driving rat sarcopenia. Mol Cell Biol. 2013;33:194–212. https://doi.org/10.1128/mcb.01036-12.
Deschenes MR, Gaertner JR, O’Reilly S. The effects of sarcopenia on muscles with different recruitment patterns and myofiber profiles. Curr Aging Sci. 2013;6:266–72. https://doi.org/10.2174/18746098113066660035.
Rygiel KA, Picard M, Turnbull DM. The ageing neuromuscular system and sarcopenia: a mitochondrial perspective. J Physiol. 2016;594:4499–512. https://doi.org/10.1113/JP271212.
Hepple RT, Rice CL. Innervation and neuromuscular control in ageing skeletal muscle. J Physiol. 2016;594:1965–78. https://doi.org/10.1113/JP270561.
Delmonico MJ, Harris TB, Visser M, Park SW, Conroy MB, Velasquez-Mieyer P, et al. Longitudinal study of muscle strength, quality, and adipose tissue infiltration. Am J Clin Nutr. 2009;90:1579–85. https://doi.org/10.3945/ajcn.2009.28047.
Mitchell WK, Williams J, Atherton P, Larvin M, Lund J, Narici M. Sarcopenia, dynapenia, and the impact of advancing age on human skeletal muscle size and strength; a quantitative review. Front Physiol. 2012;3:260. https://doi.org/10.3389/fphys.2012.00260.
Santilli V, Bernetti A, Mangone M, Paoloni M. Clinical definition of sarcopenia. Clin Cases Miner Bone Metab. 2014;11:177–80.
Hayot M, Michaud A, Koechlin C, Caron M-A, LeBlanc P, Préfaut C, et al. Skeletal muscle microbiopsy: a validation study of a minimally invasive technique. Eur Respir J. 2005;25:431–40. https://doi.org/10.1183/09031936.05.00053404.
Baguet A, Everaert I, Hespel P, Petrovic M, Achten E, Derave W. A new method for non-invasive estimation of human muscle fiber type composition. PLoS ONE. 2011;6:e21956. https://doi.org/10.1371/journal.pone.0021956.
Rodríguez Cruz PM, Cossins J, Beeson D, Vincent A. The neuromuscular junction in health and disease: molecular mechanisms governing synaptic formation and homeostasis. Front Mol Neurosci. 2020;13:610964. https://doi.org/10.3389/fnmol.2020.610964.
Ham DJ, Rüegg MA. Causes and consequences of age-related changes at the neuromuscular junction. Curr Opin Physiol. 2018;4:32–9. https://doi.org/10.1016/j.cophys.2018.04.007.
Mukund K, Subramaniam S. Skeletal muscle: a review of molecular structure and function, in health and disease. Wiley Interdiscip Rev Syst Biol Med. 2020;12:e1462. https://doi.org/10.1002/wsbm.1462.
Lepore E, Casola I, Dobrowolny G, Musarò A. Neuromuscular junction as an entity of nerve-muscle communication. Cells. 2019;8:906. https://doi.org/10.3390/cells8080906.
Rudolf R, Khan MM, Labeit S, Deschenes MR. Degeneration of neuromuscular junction in age and dystrophy. Front Aging Neurosci. 2014;6:00099. https://doi.org/10.3389/fnagi.2014.00099.
Punga AR, Ruegg MA. Signaling and aging at the neuromuscular synapse: lessons learnt from neuromuscular diseases. Curr Opin Pharmacol. 2012;12:340–6. https://doi.org/10.1016/j.coph.2012.02.002.
Ham DJ, Börsch A, Lin S, Thürkauf M, Weihrauch M, Reinhard JR, et al. The neuromuscular junction is a focal point of mTORC1 signaling in sarcopenia. Nat Commun. 2020;11:4510. https://doi.org/10.1038/s41467-020-18140-1.
Valdez G, Tapia JC, Kang H, Clemenson GD, Gage FH, Lichtman JW, et al. Attenuation of age-related changes in mouse neuromuscular synapses by caloric restriction and exercise. Proc Natl Acad Sci U S A. 2010;107:14863–8. https://doi.org/10.1073/pnas.1002220107.
Jones RA, Harrison C, Eaton SL, Llavero Hurtado M, Graham LC, Alkhammash L, et al. Cellular and molecular anatomy of the human neuromuscular junction. Cell Rep. 2017;21:2348–56. https://doi.org/10.1016/j.celrep.2017.11.008.
Wokke JHJ, Jennekens FGI, van den Oord CJM, Veldman H, Smit LME, Leppink GJ. Morphological changes in the human end plate with age. J Neurol Sci. 1990;95:291–310. https://doi.org/10.1016/0022-510X(90)90076-Y.
Oda K. Age changes of motor innervation and acetylcholine receptor distribution on human skeletal muscle fibres. J Neurol Sci. 1984;66:327–38. https://doi.org/10.1016/0022-510X(84)90021-2.
da Orssatto LBR, Wiest MJ, Diefenthaeler F. Neural and musculotendinous mechanisms underpinning age-related force reductions. Mech Ageing Dev. 2018;175:17–23. https://doi.org/10.1016/j.mad.2018.06.005.
Roberts BM, Lavin KM, Many GM, Thalacker-Mercer A, Merritt EK, Bickel CS, et al. Human neuromuscular aging: sex differences revealed at the myocellular level. Exp Gerontol. 2018;106:116–24. https://doi.org/10.1016/j.exger.2018.02.023.
St-Jean-Pelletier F, Pion CH, Leduc-Gaudet JP, Sgarioto N, Zovilé I, Barbat-Artigas S, et al. The impact of ageing, physical activity, and pre-frailty on skeletal muscle phenotype, mitochondrial content, and intramyocellular lipids in men. J Cachexia Sarcopenia Muscle. 2017;8:213–28. https://doi.org/10.1002/jcsm.12139.
Kwon YN, Yoon SS. Sarcopenia: neurological point of view. J Bone Metab. 2017;24:83–9. https://doi.org/10.11005/jbm.2017.24.2.83.
Joanisse S, Nederveen JP, Snijders T, McKay BR, Parise G. Skeletal muscle regeneration, repair and remodelling in aging: the importance of muscle stem cells and vascularization. Gerontology. 2016;63:91–100. https://doi.org/10.1159/000450922.
Li M, Larsson L. Force-generating capacity of human myosin isoforms extracted from single muscle fibre segments. J Physiol. 2010;588:5105–14. https://doi.org/10.1113/jphysiol.2010.199067.
Verdijk LB, Snijders T, Beelen M, Savelberg HHCM, Meijer K, Kuipers H, et al. Characteristics of muscle fiber type are predictive of skeletal muscle mass and strength in elderly men. J Am Geriatr Soc. 2010;58:2069–75. https://doi.org/10.1111/j.1532-5415.2010.03150.x.
Covault J, Sanes JR. Neural cell adhesion molecule (N-CAM) accumulates in denervated and paralyzed skeletal muscles. Proc Natl Acad Sci U S A. 1985;82:4544–8. https://doi.org/10.1073/pnas.82.13.4544.
Hendrickse P, Galinska M, Hodson-Tole E, Degens H. An evaluation of common markers of muscle denervation in denervated young-adult and old rat gastrocnemius muscle. Exp Gerontol. 2018;106:159–64. https://doi.org/10.1016/j.exger.2018.03.007.
Gillon A, Sheard P. Elderly mouse skeletal muscle fibres have a diminished capacity to upregulate NCAM production in response to denervation. Biogerontology. 2015;16:811–23. https://doi.org/10.1007/s10522-015-9608-6.
Soendenbroe C, Heisterberg MF, Schjerling P, Karlsen A, Kjaer M, Andersen JL, et al. Molecular indicators of denervation in aging human skeletal muscle. Muscle Nerve. 2019;60:453–63. https://doi.org/10.1002/mus.26638.
Edström E, Ulfhake B. Sarcopenia is not due to lack of regenerative drive in senescent skeletal muscle. Aging Cell. 2005;4:65–77. https://doi.org/10.1111/j.1474-9728.2005.00145.x.
Chen J, Mizushige T, Nishimune H. Active zone density is conserved during synaptic growth but impaired in aged mice. J Comp Neurol. 2012;520:434–52. https://doi.org/10.1002/cne.22764.
Hurtado E, Cilleros V, Nadal L, Simó A, Obis T, Garcia N, et al. Muscle contraction regulates BDNF/TrkB signaling to modulate synaptic function through presynaptic cPKCα and cPKCβi. Front Mol Neurosci. 2017;10:147. https://doi.org/10.3389/fnmol.2017.00147.
Nishimune H, Numata T, Chen J, Aoki Y, Wang Y, Starr MP, et al. Active zone protein Bassoon co-localizes with presynaptic calcium channel, modifies channel function, and recovers from aging related loss by exercise. PLoS ONE. 2012;7:e38029. https://doi.org/10.1371/journal.pone.0038029.
Ross JA, Webster RG, Lechertier T, Reynolds LE, Turmaine M, Bencze M, et al. Multiple roles of integrin-α3 at the neuromuscular junction. J Cell Sci. 2017;130:1772–84. https://doi.org/10.1242/jcs.201103.
Chen J, Billings SE, Nishimune H. Calcium channels link the muscle-derived synapse organizer laminin β2 to Bassoon and CAST/Erc2 to organize presynaptic active zones. J Neurosci. 2011;31:512–25. https://doi.org/10.1523/JNEUROSCI.3771-10.2011.
Waites CL, Leal-Ortiz SA, Okerlund N, Dalke H, Fejtova A, Altrock WD, et al. Bassoon and Piccolo maintain synapse integrity by regulating protein ubiquitination and degradation. EMBO J. 2013;32:954–69. https://doi.org/10.1038/emboj.2013.27.
Ivanova D, Dirks A, Fejtova A. Bassoon and piccolo regulate ubiquitination and link presynaptic molecular dynamics with activity-regulated gene expression. J Physiol. 2016;594:5441–8. https://doi.org/10.1113/JP271826.
Shi L, Fu AKY, Ip NY. Molecular mechanisms underlying maturation and maintenance of the vertebrate neuromuscular junction. Trends Neurosci. 2012;35:441–53. https://doi.org/10.1016/j.tins.2012.04.005.
Nishimune H, Badawi Y, Mori S, Shigemoto K. Dual-color STED microscopy reveals a sandwich structure of Bassoon and Piccolo in active zones of adult and aged mice. Sci Rep. 2016;6:27. https://doi.org/10.1038/srep27935.
Casati M, Costa AS, Capitanio D, Ponzoni L, Ferri E, Agostini S, et al. The biological foundations of sarcopenia: established and promising markers. Front Med. 2019;6:184. https://doi.org/10.3389/fmed.2019.00184.
Antonucci F, Corradini I, Fossati G, Tomasoni R, Menna E, Matteoli M. SNAP-25, a known presynaptic protein with emerging postsynaptic functions. Front Synaptic Neurosci. 2016;8:7. https://doi.org/10.3389/fnsyn.2016.00007.
Simó A, Cilleros-Mañé V, Just-Borràs L, Hurtado E, Nadal L, Tomàs M, et al. nPKCε mediates SNAP-25 phosphorylation of Ser-187 in basal conditions and after synaptic activity at the neuromuscular junction. Mol Neurobiol. 2019;56:5346–64. https://doi.org/10.1007/s12035-018-1462-5.
Islamov RR, Samigullin DV, Rizvanov AA, Bondarenko NI, Nikolskiy EE. Synaptosome-associated protein 25 (SNAP25) synthesis in terminal buttons of mouse motor neuron. Dokl Biochem Biophys. 2015;464:272–4. https://doi.org/10.1134/S1607672915050026.
Giniatullin AR, Darios F, Shakirzyanova A, Davletov B, Giniatullin R. SNAP25 is a pre-synaptic target for the depressant action of reactive oxygen species on transmitter release. J Neurochem. 2006;98:1789–97. https://doi.org/10.1111/j.1471-4159.2006.03997.x.
Kaneai N, Arai M, Takatsu H, Fukui K, Urano S. Vitamin E inhibits oxidative stress-induced denaturation of nerve terminal proteins involved in neurotransmission. J Alzheimer’s Dis. 2012;28:183–9. https://doi.org/10.3233/JAD-2011-111133.
Liguori I, Russo G, Curcio F, Bulli G, Aran L, Della-Morte D, et al. Oxidative stress, aging, and diseases. Clin Interv Aging. 2018;13:757–72. https://doi.org/10.2147/CIA.S158513.
Baumann CW, Kwak D, Liu HM, Thompson LV. Age-induced oxidative stress: how does it influence skeletal muscle quantity and quality? J Appl Physiol. 2016;121:1047–52. https://doi.org/10.1152/japplphysiol.00321.2016.
Kalinkovich A, Livshits G. Sarcopenia - the search for emerging biomarkers. Ageing Res Rev. 2015;22:58–71. https://doi.org/10.1016/j.arr.2015.05.001.
Uchitomi R, Hatazawa Y, Senoo N, Yoshioka K, Fujita M, Shimizu T, et al. Metabolomic analysis of skeletal muscle in aged mice. Sci Rep. 2019;9:10425. https://doi.org/10.1038/s41598-019-46929-8.
Sugita S, Fleming LL, Wood C, Vaughan SK, Gomes MPSM, Camargo W, et al. VAChT overexpression increases acetylcholine at the synaptic cleft and accelerates aging of neuromuscular junctions. Skelet Muscle. 2016;6:31. https://doi.org/10.1186/s13395-016-0105-7.
Vaughan SK, Sutherland NM, Valdez G. Attenuating cholinergic transmission increases the number of satellite cells and preserves muscle mass in old age. Front Aging Neurosci. 2019;11:262. https://doi.org/10.3389/fnagi.2019.00262.
Cetin H, Beeson D, Vincent A, Webster R. The structure, function, and physiology of the fetal and adult acetylcholine receptor in muscle. Front Mol Neurosci. 2020;13:581097. https://doi.org/10.3389/fnmol.2020.581097.
Bao Z, Cui C, Chow SK-H, Qin L, Wong RMY, Cheung W-H. AChRs degeneration at NMJ in aging-associated sarcopenia – a systematic review. Front Aging Neurosci. 2020;12:597811. https://doi.org/10.3389/fnagi.2020.597811.
Soendenbroe C, Bechshøft CJL, Heisterberg MF, Jensen SM, Bomme E, Schjerling P, et al. Key components of human myofibre denervation and neuromuscular junction stability are modulated by age and exercise. Cells. 2020;9:893. https://doi.org/10.3390/cells9040893.
Witzemann V, Brenner H-R, Sakmann B. Neural factors regulate AChR subunit mRNAs at rat neuromuscular synapses. J Cell Biol. 1991;114:125–41. https://doi.org/10.1083/jcb.114.1.125.
Caron M-A, Charette SJ, Maltais F, Debigaré R. Variability of protein level and phosphorylation status caused by biopsy protocol design in human skeletal muscle analyses. BMC Res Notes. 2011;4:488. https://doi.org/10.1186/1756-0500-4-488.
Apel PJ, Alton T, Northam C, Ma J, Callahan M, Sonntag WE, et al. How age impairs the response of the neuromuscular junction to nerve transection and repair: an experimental study in rats. J Orthop Res. 2009;27:385–93. https://doi.org/10.1002/jor.20773.
Aare S, Spendiff S, Vuda M, Elkrief D, Perez A, Wu Q, et al. Failed reinnervation in aging skeletal muscle. Skelet Muscle. 2016;6:29. https://doi.org/10.1186/s13395-016-0101-y.
Zhao K, Shen C, Li L, Wu H, Xing G, Dong Z, et al. Sarcoglycan alpha mitigates neuromuscular junction decline in aged mice by stabilizing LRP4. J Neurosci. 2018;38:8860–73. https://doi.org/10.1523/JNEUROSCI.0860-18.2018.
Cisterna BA, Vargas AA, Puebla C, Fernández P, Escamilla R, Lagos CF, et al. Active acetylcholine receptors prevent the atrophy of skeletal muscles and favor reinnervation. Nat Commun. 2020;11:1073. https://doi.org/10.1038/s41467-019-14063-8.
Ma J, Shen J, Garrett JP, Lee CA, Li Z, Elsaidi GA, et al. Gene expression of myogenic regulatory factors, nicotinic acetylcholine receptor subunits, and GAP-43 in skeletal muscle following denervation in a rat model. J Orthop Res. 2007;25:1498–505. https://doi.org/10.1002/jor.20414.
Chen A, Bai L, Zhong K, Shu X, Wang A, Xiao Y, et al. APC2CDH1 negatively regulates agrin signaling by promoting the ubiquitination and proteolytic degradation of DOK7. FASEB J. 2020;34:12009–23. https://doi.org/10.1096/fj.202000485R.
Rimer M. Emerging roles for MAP kinases in agrin signaling. Commun Integr Biol. 2011;4:143–6. https://doi.org/10.4161/psb.4.2.14357.
Landi F, Calvani R, Lorenzi M, Martone AM, Tosato M, Drey M, et al. Serum levels of C-terminal agrin fragment (CAF) are associated with sarcopenia in older multimorbid community-dwellers: results from the ilSIRENTE study. Exp Gerontol. 2016;79:31–6. https://doi.org/10.1016/j.exger.2016.03.012.
Ohno K, Ohkawara B, Ito M. Agrin-LRP4-MuSK signaling as a therapeutic target for myasthenia gravis and other neuromuscular disorders. Expert Opin Ther Targets. 2017;21:949–58. https://doi.org/10.1080/14728222.2017.1369960.
Nishimune H, Shigemoto K. Pratical anatomy of the neuromuscular junction in health and disease. Neurol Clin. 2018;36:231–40. https://doi.org/10.1016/j.ncl.2018.01.009.
Naguib M, Flood P, McArdle JJ, Brenner HR. Advances in neurobiology of the neuromuscular junction: implications for the anesthesiologist. Anesthesiology. 2002;96:202–31. https://doi.org/10.1097/00000542-200201000-00035.
Blasco A, Gras S, Mòdol-Caballero G, Tarabal O, Casanovas A, Piedrafita L, et al. Motoneuron deafferentation and gliosis occur in association with neuromuscular regressive changes during ageing in mice. J Cachexia Sarcopenia Muscle. 2020;11:1628–60. https://doi.org/10.1002/jcsm.12599.
Dunne V, Maselli RA. Identification of pathogenic mutations in the human rapsyn gene. J Hum Genet. 2003;48:204–7. https://doi.org/10.1007/s10038-003-0005-7.
Bolliger MF, Zurlinden A, Lüscher D, Bütikofer L, Shakhova O, Francolini M, et al. Specific proteolytic cleavage of agrin regulates maturation of the neuromuscular junction. J Cell Sci. 2010;123:3944–55. https://doi.org/10.1242/jcs.072090.
Reif R, Sales S, Hettwer S, Dreier B, Gisler C, Wölfel J, et al. Specific cleavage of agrin by neurotrypsin, a synaptic protease linked to mental retardation. FASEB J. 2007;21:3468–78. https://doi.org/10.1096/fj.07-8800com.
Hettwer S, Dahinden P, Kucsera S, Farina C, Ahmed S, Fariello R, et al. Elevated levels of a C-terminal agrin fragment identifies a new subset of sarcopenia patients. Exp Gerontol. 2013;48:69–75. https://doi.org/10.1016/j.exger.2012.03.002.
Marzetti E, Calvani R, Lorenzi M, Marini F, D’Angelo E, Martone AM, et al. Serum levels of C-terminal agrin fragment (CAF) are associated with sarcopenia in older hip fractured patients. Exp Gerontol. 2014;60:79–82. https://doi.org/10.1016/j.exger.2014.10.003.
Bütikofer L, Zurlinden A, Bolliger MF, Kunz B, Sonderegger P. Destabilization of the neuromuscular junction by proteolytic cleavage of agrin results in precocious sarcopenia. FASEB J. 2011;25:4378–93. https://doi.org/10.1096/fj.11-191262.
Hettwer S, Lin S, Kucsera S, Haubitz M, Oliveri F, Fariello RG, et al. Injection of a soluble fragment of neural agrin (NT-1654) considerably improves the muscle pathology caused by the disassembly of the neuromuscular junction. PLoS ONE. 2014;9:e88739. https://doi.org/10.1371/journal.pone.0088739.
Colombo E, Bedogni F, Lorenzetti I, Landsberger N, Previtali SC, Farina C. Autocrine and immune cell-derived BDNF in human skeletal muscle: implications for myogenesis and tissue regeneration. J Pathol. 2013;231:190–8. https://doi.org/10.1002/path.4228.
Sakuma K, Yamaguchi A. The recent understanding of the neurotrophin’s role in skeletal muscle adaptation. J Biomed Biotechnol. 2011;2011:201696. https://doi.org/10.1155/2011/201696.
Leßmann V, Brigadski T. Mechanisms, locations, and kinetics of synaptic BDNF secretion: an update. Neurosci Res. 2009;65:11–22. https://doi.org/10.1016/j.neures.2009.06.004.
Greising SM, Stowe JM, Sieck GC, Mantilla CB. Role of TrkB kinase activity in aging diaphragm neuromuscular junctions. Exp Gerontol. 2015;72:184–91. https://doi.org/10.1016/j.exger.2015.10.013.
Miyazaki S, Iino N, Koda R, Narita I, Kaneko Y. Brain-derived neurotrophic factor is associated with sarcopenia and frailty in Japanese hemodialysis patients. Geriatr Gerontol Int. 2020. https://doi.org/10.1111/ggi.14089.
Shimada H, Makizako H, Doi T, Yoshida D, Tsutsumimoto K, Anan Y, et al. A large, cross-sectional observational study of serum BDNF, cognitive function, and mild cognitive impairment in the elderly. Front Aging Neurosci. 2014;6:69. https://doi.org/10.3389/fnagi.2014.00069.
Kwak JY, Hwang H, Kim S-K, Choi JY, Lee S-M, Bang H, et al. Prediction of sarcopenia using a combination of multiple serum biomarkers. Sci Rep. 2018;8:8574. https://doi.org/10.1038/s41598-018-26617-9.
Gómez-Pinilla F, Ying Z, Roy RR, Molteni R, Edgerton VR. Voluntary exercise induces a BDNF-mediated mechanism that promotes neuroplasticity. J Neurophysiol. 2002;88:2187–95. https://doi.org/10.1152/jn.00152.2002.
Matthews VB, Åström MB, Chan MHS, Bruce CR, Krabbe KS, Prelovsek O, et al. Brain-derived neurotrophic factor is produced by skeletal muscle cells in response to contraction and enhances fat oxidation via activation of AMP-activated protein kinase. Diabetologia. 2009;52:1409–18. https://doi.org/10.1007/s00125-009-1364-1.
Gomes M, Figueiredo D, Teixeira L, Poveda V, Paúl C, Santos-Silva A, et al. Physical inactivity among older adults across Europe based on the SHARE database. Age Ageing. 2017;46:71–7. https://doi.org/10.1093/ageing/afw165.
Nagano M, Suzuki H. Quantitative analyses of expression of GDNF and neurotrophins during postnatal development in rat skeletal muscles. Neurosci Res. 2003;45:391–9. https://doi.org/10.1016/S0168-0102(03)00010-5.
McCullough MJ, Peplinski NG, Kinnell KR, Spitsbergen JM. Glial cell line-derived neurotrophic factor (GDNF) protein content in rat skeletal muscle is altered by increased physical activity in vivo and in vitro. Neuroscience. 2011;174:234–44. https://doi.org/10.1016/j.neuroscience.2010.11.016.
Victoria Vega A, Avila G. CGRP, a vasodilator neuropeptide that stimulates neuromuscular transmission and EC coupling. Curr Vasc Pharmacol. 2010;8:394–403. https://doi.org/10.2174/157016110791112287.
Buffelli M, Pasino E, Cangiano A. In vivo acetylcholine receptor expression induced by calcitonin gene-related peptide in rat soleus muscle. Neuroscience. 2001;104:561–7. https://doi.org/10.1016/S0306-4522(01)00090-2.
Parnow A, Gharakhanlou R, Gorginkaraji Z, Rajabi S, Eslami R, Hedayati M, et al. Effects of endurance and resistance training on calcitonin gene-related peptide and acetylcholine receptor at slow and fast twitch skeletal muscles and sciatic nerve in male wistar rats. Int J Pept. 2012;2012:962651. https://doi.org/10.1155/2012/962651.
Machado J, Manfredi LH, Silveira WA, Gonçalves DAP, Lustrino D, Zanon NM, et al. Calcitonin gene-related peptide inhibits autophagic-lysosomal proteolysis through cAMP/PKA signaling in rat skeletal muscles. Int J Biochem Cell Biol. 2016;72:40–50. https://doi.org/10.1016/j.biocel.2015.12.011.
Matteoli M, Balbi S, Sala C, Chini B, Cimino M, Vitadello M, et al. Developmentally regulated expression of calcitonin gene-related peptide at mammalian neuromuscular junction. J Mol Neurosci. 1990;2:175–84. https://doi.org/10.1007/BF02896842.
Tarabal O. Regulation of motoneuronal calcitonin gene-related peptide (CGRP) during axonal growth and neuromuscular synaptic plasticity induced by botulinum toxin in rats. Eur J Neurosci. 1996;8:829–36. https://doi.org/10.1111/j.1460-9568.1996.tb01269.x.
Machado J, Silveira WA, Gonçalves DA, Schavinski AZ, Khan MM, Zanon NM, et al. α−calcitonin gene-related peptide inhibits autophagy and calpain systems and maintains the stability of neuromuscular junction in denervated muscles. Mol Metab. 2019;28:91–106. https://doi.org/10.1016/j.molmet.2019.06.024.
Baraldo M, Geremia A, Pirazzini M, Nogara L, Solagna F, Türk C, et al. Skeletal muscle mTORC1 regulates neuromuscular junction stability. J Cachexia Sarcopenia Muscle. 2020;11:208–25. https://doi.org/10.1002/jcsm.12496.
Joseph GA, Wang SX, Jacobs CE, Zhou W, Kimble GC, Tse HW, et al. Partial inhibition of mTORC1 in aged rats counteracts the decline in muscle mass and reverses molecular signaling associated with sarcopenia. Mol Cell Biol. 2019;39:e00141-e219. https://doi.org/10.1128/MCB.00141-19.
Edwards BJ, Perry HM, Kaiser FE, Morley JE, Kraenzle D, Stevenson R, et al. Relationship of age and calcitonin gene-related peptide to postprandial hypotension. Mech Ageing Dev. 1996;87:61–73. https://doi.org/10.1016/0047-6374(96)01688-0.
Holahan MR. A shift from a pivotal to supporting role for the growth-associated protein (GAP-43) in the coordination of axonal structural and functional plasticity. Front Cell Neurosci. 2017;11:1–19. https://doi.org/10.3389/fncel.2017.00266.
Hesselmans LFGM, Jennekens FGI, van den Oord CJM, Oestreicher AB, Veldman H, Gispen WH. A light and electron microscopical study of B-50 (GAP-43) in human intramuscular nerve and neuromuscular junctions during development. J Neurol Sci. 1989;89:301–11. https://doi.org/10.1016/0022-510X(89)90031-2.
Aigner L, Arber S, Kapfhammer JP, Laux T, Schneider C, Botteri F, et al. Overexpression of the neural growth-associated protein GAP-43 induces nerve sprouting in the adult nervous system of transgenic mice. Cell. 1995;83:269–78. https://doi.org/10.1016/0092-8674(95)90168-X.
Woolf CJ, Reynolds ML, Chong MS, Emson P, Irwin N, Benowitz LI. Denervation of the motor endplate results in the rapid expression by terminal Schwann cells of the growth-associated protein GAP-43. J Neurosci. 1992;12:3999–4010. https://doi.org/10.1523/jneurosci.12-10-03999.1992.
Caprara GA, Morabito C, Perni S, Navarra R, Guarnieri S, Mariggiò MA. Evidence for altered Ca2+ handling in growth associated protein 43-knockout skeletal muscle. Front Physiol. 2016;7:493. https://doi.org/10.3389/fphys.2016.00493.
Guarnieri S, Morabito C, Paolini C, Boncompagni S, Pilla R, Fanò-Illic G, et al. Growth associated protein 43 is expressed in skeletal muscle fibers and is localized in proximity of mitochondria and calcium release units. PLoS ONE. 2013;8:e53267. https://doi.org/10.1371/journal.pone.0053267.
Johnson H, Mossberg K, Arvidsson U, Piehl F, Hökfelt T, Ulfhake B. Increase in α-CGRP and GAP-43 in aged motoneurons: a study of peptides, growth factors, and ChAT mRNA in the lumbar spinal cord of senescent rats with symptoms of hindlimb incapacities. J Comp Neurol. 1995;359:69–89. https://doi.org/10.1002/cne.903590106.
Verzè L, Buffo A, Rossi F, Oestreicher AB, Gispen WH, Strata P. Increase of B-50/GAP-43 immunoreactivity in uninjured muscle nerves of MDX mice. Neuroscience. 1996;70:807–15. https://doi.org/10.1016/S0306-4522(96)83017-X.
Heuß D, Engelhardt A, Göbel H, Neundörfer B. Light-microscopic study of phosphoprotein B-50 in myopathies. Virchows Arch. 1995;426:69–76. https://doi.org/10.1007/BF00194700.
Yoshimoto Y, Ikemoto-Uezumi M, Hitachi K, Fukada S, Uezumi A. Methods for accurate assessment of myofiber maturity during skeletal muscle regeneration. Front Cell Dev Biol. 2020;8:267. https://doi.org/10.3389/fcell.2020.00267.
Sousa-Victor P, García-Prat L, Muñoz-Cánoves P. Control of satellite cell function in muscle regeneration and its disruption in ageing. Nat Rev Mol Cell Biol. 2021. https://doi.org/10.1038/s41580-021-00421-2.
Sajko Š, Kubínová L, Cvetko E, Kreft M, Wernig A, Eržen I. Frequency of M-cadherin-stained satellite cells declines in human muscles during aging. J Histochem Cytochem. 2004;52:179–85. https://doi.org/10.1177/002215540405200205.
Shefer G, Van de Mark DP, Richardson JB, Yablonka-Reuveni Z. Satellite-cell pool size does matter: defining the myogenic potency of aging skeletal muscle. Dev Biol. 2006;294:50–66. https://doi.org/10.1016/j.ydbio.2006.02.022.
Arpke RW, Shams AS, Collins BC, Larson AA, Lu N, Lowe DA, et al. Preservation of satellite cell number and regenerative potential with age reveals locomotory muscle bias. Skelet Muscle. 2021;11:22. https://doi.org/10.1186/s13395-021-00277-2.
Collins CA, Zammit PS, Ruiz AP, Morgan JE, Partridge TA. A population of myogenic stem cells that survives skeletal muscle aging. Stem Cells. 2007;25:885–94. https://doi.org/10.1634/stemcells.2006-0372.
Sousa-Victor P, Gutarra S, García-Prat L, Rodriguez-Ubreva J, Ortet L, Ruiz-Bonilla V, et al. Geriatric muscle stem cells switch reversible quiescence into senescence. Nature. 2014;506:316–21. https://doi.org/10.1038/nature13013.
Liu W, Klose A, Forman S, Paris ND, Wei-LaPierre L, Cortés-Lopéz M, et al. Loss of adult skeletal muscle stem cells drives age-related neuromuscular junction degeneration. Elife. 2017;6:e26464. https://doi.org/10.7554/eLife.26464.
Liu W, Wei-LaPierre L, Klose A, Dirksen RT, Chakkalakal JV. Inducible depletion of adult skeletal muscle stem cells impairs the regeneration of neuromuscular junctions. Elife. 2015;4:e09221. https://doi.org/10.7554/eLife.09221.
Snijders T, Nederveen JP, McKay BR, Joanisse S, Verdijk LB, van Loon LJC, et al. Satellite cells in human skeletal muscle plasticity. Front Physiol. 2015;6:283. https://doi.org/10.3389/fphys.2015.00283.
Funding
This work was supported by CIAFEL (UIDB/00617/2020), LAQV (UIDB/50006/2020), and CITAB (UIDB/04033/2020) research units and by A.M.P.’s fellowship (SFRH/BD/144396/2019) through national funds by the Portuguese Foundation for Science and Technology (FCT) and co-financed by the European Regional Development Fund (FEDER), within the PT2020 Partnership Agreement.
Author information
Authors and Affiliations
Contributions
A.M.P. conducted the literature search and drafted the manuscript, and R.F., P.A.O., and J.A.D. critically revised the work.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Moreira-Pais, A., Ferreira, R., Oliveira, P. et al. A neuromuscular perspective of sarcopenia pathogenesis: deciphering the signaling pathways involved. GeroScience 44, 1199–1213 (2022). https://doi.org/10.1007/s11357-021-00510-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11357-021-00510-2