Abstract
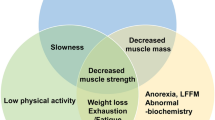
Healthy aging is the individual’s possibility of maintaining the functional ability that enables well-being in older age, along with the maintenance of independence, a crucial factor for ensuring health and quality of life in older people. Loss of muscle mass, strength, and function are relevant threats to independent living and progressively occur with senescence and aging, worsening physical performance, and thus characterizing sarcopenia. Identifying sarcopenia in clinical practice is essential for the early onset of treatment minimizing its impacts and thus improving the patient’s quality of life. The purpose of this work was to conduct a literature review of the pathophysiological and molecular mechanisms underlying muscle changes during aging, highlighting the role of oxidative stress, cytokines, and inflammation, as well as strategies to prevent and treat age-related muscle alterations.




Similar content being viewed by others
References
Beard JR, Officer A, de Carvalho IA, et al. The World report on ageing and health: a policy framework for healthy ageing. Lancet. 2016;387(10033):2145–154. https://doi.org/10.1016/S0140-6736(15)00516-4.
Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyère O, Cederholm T, et al. Consensus definition of sarcopenia, cachexia and pre-cachexia: Joint document elaborated by Special Interest Groups (SIG) “cachexia-anorexia in chronic wasting diseases” and “nutrition in geriatrics.”. Age Ageing. 2018;29:1–16.
Goisser S, Kemmler W, Porzel S, Volkert D, Sieber CC, Bollheimer LC, et al. Sarcopenic obesity and complex interventions with nutrition and exercise in community-dwelling older persons--a narrative review. Clin Interv Aging. 2015;10:1267–82.
Wolfe RR. The role of dietary protein in optimizing muscle mass, function and health outcomes in older individuals. Br J Nutr. 2012;108(S2):S88–93.
Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, et al. Sarcopenia: European consensus on definition and diagnosis: report of the European working group on sarcopenia in older people. Age Ageing. 2010;39(4):412–23.
Vellas B, Fielding R, Bens C, Bernabei R, Cawthon P, Cederholm T, et al. Implications of ICD-10 for sarcopenia clinical practice and clinical trials: report by the International Conference on Frailty and Sarcopenia Research Task Force. J Frailty Aging. 2018;7(1):2–9.
Baumgartner RN, Koehler KM, Gallagher D, Romero L, Heymsfield SB, Ross RR, et al. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol. 1998;147(8):755–63.
Iannuzzi-Sucich M, Prestwood KM, Kenny AM. Prevalence of sarcopenia and predictors of skeletal muscle mass in healthy, older men and women. J Gerontol A Biol Sci Med Sci. 2002;57(12):M772–7.
Smoliner C, Sieber CC, Wirth R. Prevalence of sarcopenia in geriatric hospitalized patients. J Am Med Dir Assoc. 2014;15(4):267–72.
Alexandre TDS, Duarte YADO, Santos JLF, Wong R, Lebrão ML. Prevalence and associated factors of sarcopenia among elderly in Brazil: findings from the sabe study. J Nutr Health Aging. 2014;18(3):284–90.
Zhang X, Zhang W, Wang C, Tao W, Dou Q, Yang Y. Sarcopenia as a predictor of hospitalization among older people: a systematic review and meta-analysis. BMC Geriatr. 2018;18(1):188.
Cruz-Jentoft AJ, Landi F, Topinková E, Michel JP. Understanding sarcopenia as a geriatric syndrome. Curr Opin Clin Nutr Metab Care. 2010;13(1):1–7.
Carvalho do Nascimento PR, Poitras S, Bilodeau M. How do we define and measure sarcopenia? Protocol for a systematic review. Syst Rev. 2018;7(1):51. https://doi.org/10.1186/s13643-018-0712-y.
Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyère O, Cederholm T, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019;48(1):16–31.
Malmstrom TK, Morley JE. SARC-F: a simple questionnaire to rapidly diagnose sarcopenia. J Am Med Dir Assoc. 2013;14(8):531–2.
Ida S, Kaneko R, Murata K. SARC-F for screening of sarcopenia among older adults: a meta-analysis of screening test accuracy. J Am Med Dir Assoc. 2018;19(8):685–9.
Bahat G, Yilmaz O, Kiliç C, Oren MM, Karan MA. Performance of SARC-F in regard to sarcopenia definitions, muscle mass and functional measures. J Nutr Health Aging. 2018;22(8):898–903.
Ellis KJ. Human body composition: in vivo methods. Physiol Rev. 2000;80(2):649–80.
Silva AM, Shen W, Heo M, Gallagher D, Wang Z, Sardinha LB, et al. Ethnicity-related skeletal muscle differences across the lifespan. Am J Hum Biol. 2010;22(1):76–82.
Heymsfield SB, Peterson CM, Thomas DM, Heo M, Schuna JM, Hong S, et al. Scaling of adult body weight to height across sex and race/ethnic groups: relevance to BMI. Am J Clin Nutr. 2014;100(6):1455–61.
Thomas D, Das SK, Levine JA, Martin CK, Mayer L, McDougall A, et al. New fat free mass - fat mass model for use in physiological energy balance equations. Nutr Metab (Lond). 2010;7:39.
McNally EM. Powerful genes — myostatin regulation of human muscle mass. N Engl J Med. 2004;350(26):2642–4.
Heymsfield SB, Scherzer R, Pietrobelli A, Lewis CE, Grunfeld C. Body mass index as a phenotypic expression of adiposity: quantitative contribution of muscularity in a population-based sample. Int J Obes. 2009;33(12):1363–73.
Bhasin S, Cunningham GR, Hayes FJ, Matsumoto AM, Snyder PJ, Swerdloff RS, et al. Testosterone therapy in adult men with androgen deficiency syndromes : an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2010;95(6):2536–59.
Houston DK, Nicklas BJ, Ding J, Harris TB, Tylavsky FA, Newman AB, et al. Dietary protein intake is associated with lean mass change in older, community-dwelling adults: the Health, Aging, and Body Composition (Health ABC) Study. Am J Clin Nutr. 2008;87(1):150–5.
Lee SY, Gallagher D. Assessment methods in human body composition. Curr Opin Clin Nutr Metab Care. 2008;11(5):566–72. https://doi.org/10.1097/MCO.0b013e32830b5f23.
Kim KM, Jang HC, Lim S. Differences among skeletal muscle mass indices derived from height-, weight-, and body mass index-adjusted models in assessing sarcopenia. Korean J Intern Med. 2016;31(4):643–50. https://doi.org/10.3904/kjim.2016.015.
Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, et al. Sarcopenia: European consensus on definition and diagnosis. Age Ageing. 2010;39(4):412–23.
Valensise H, Andreoli A, Lello S, Magnani F, Romanini C, De Lorenzo A. Total-body skeletal muscle mass: development and cross-validation of anthropometric prediction models. Am J Clin Nutr. 2000;72(3):796–803.
Rech CR, Dellagrana RA, Marucci M d FN, Petroski EL. Validade de equações antropométricas para estimar a massa muscular em idosos. Rev Bras Cineantropometria e Desempenho Hum. 2012;14(1):23–31. https://doi.org/10.5007/1980-0037.2012v14n1p23.
Goodpaster BH, Park SW, Harris TB, Kritchevsky SB, Nevitt M, Schwartz AV, et al. The loss of skeletal muscle strength, mass, and quality in older adults: the health, aging and body composition study. J Gerontol A Biol Sci Med Sci. 2006;61(10):1059–64.
Janssen I, Baumgartner RN, Ross R, Rosenberg IH, Roubenoff R. Skeletal muscle cutpoints associated with elevated physical disability risk in older men and women. Am J Epidemiol. 2004;159:413–21.
Morley JE, Anker SD, von Haehling S. Prevalence, incidence, and clinical impact of sarcopenia: facts, numbers, and epidemiology-update 2014. J Cachexia Sarcopenia Muscle. 2014;5(4):253–9.
Deitrick JE, Whedon GD, Shorr E. Effects of immobilization upon various metabolic and physiologic functions of normal men. Am J Med. 1948;4(1):3–36.
Torres-de Araújo JR, Tomaz-de Lima RR, Ferreira-Bendassolli IM, Costa-de LK. Functional, nutritional and social factors associated with mobility limitations in the elderly: a systematic review. Salud Publica Mex. 2018;60(5):579–85.
Kalinkovich A, Livshits G. Sarcopenic obesity or obese sarcopenia: a cross talk between age-associated adipose tissue and skeletal muscle inflammation as a main mechanism of the pathogenesis. Ageing Res Rev. 2017;35:200–21.
Matsudo SM, Matsudo VKR, Neto TLDB. Impacto do envelhecimento nas variáveis antropométricas, neuromotoras e metabólicas da aptidão física. Rev Bras Ciênc Mov. 2000;8(4):21–32.
Visser M, Kritchevsky S, Goodpaster B, Newman AB, Nevitt MC, Stamm E, et al. Leg muscle mass and composition in relation to lower extremity performance in men and women aged 70 to 79: the health, aging and body composition study. J Am Geriatr Soc. 2002;50(5):897–904.
Siu PM, Pistilli EE, Alway SE. Age-dependent increase in oxidative stress in gastrocnemius muscle with unloading. J Appl Physiol. 2008;105(6):1695–705.
Cannavino J, Brocca L, Sandri M, Grassi B, Bottinelli R, Pellegrino MA. The role of alterations in mitochondrial dynamics and PGC-1α over-expression in fast muscle atrophy following hindlimb unloading. J Physiol. 2015;593(8):1981–95.
Piette J, Piret B, Bonizzi G, Schoonbroodt S, Merville MP, Legrand-Poels S, et al. Multiple redox regulation in NF-kappaB transcription factor activation. Biol Chem. 1997;378(11):1237–45.
Chung HY, Cesari M, Anton S, Marzetti E, Giovannini S, Seo AY, et al. Molecular inflammation: underpinnings of aging and age-related diseases. Ageing Res Rev. 2009;8:18–30.
Davalli P, Mitic T, Caporali A, Lauriola A, D’Arca D. ROS, cell senescence, and novel molecular mechanisms in aging and age-related diseases. Vol. 2016, Oxidative Med Cell Longev 2016.
Li H, Malhotra S, Kumar A. Nuclear factor-kappa B signaling in skeletal muscle atrophy. J Mol Med (Berl). 2008;86(10):1113–26. https://doi.org/10.1007/s00109-008-0373-8.
Rodriguez J, Vernus B, Chelh I, Cassar-Malek I, Gabillard JC, Hadj Sassi A, et al. Myostatin and the skeletal muscle atrophy and hypertrophy signaling pathways. Cell Mol Life Sci. 2014;71:4361–71.
Cai D, Frantz J, Tawa NEJ, Melendez PA, Oh BC, Lidov HG, et al. IKKβ/NF-κB activation causes severe muscle wasting in mice. Cell. 2017;119(2):285–98. Available from:. https://doi.org/10.1016/j.cell.2004.09.027.
Li YP, Schwartz RJ, Waddell ID, Holloway BR, Reid MB. Skeletal muscle myocytes undergo protein loss and reactive oxygen-mediated NF-kappaB activation in response to tumor necrosis factor alpha. FASEB J. 1998;12(10):871–80.
Dogra C, Changotra H, Wedhas N, Qin X, Wergedal JE, Kumar A. TNF-related weak inducer of apoptosis (TWEAK) is a potent skeletal muscle-wasting cytokine. FASEB J. 2007;21(8):1857–69 Available from: http://www.fasebj.org/content/21/8/1857.
Frontera WR, Ochala J. Skeletal muscle: a brief review of structure and function. Calcif Tissue Int. 2015;96(3):183–95 Available from: http://link.springer.com/10.1007/s00223-014-9915-y.
Schiaffino S, Reggiani C. Fiber types in mammalian skeletal muscles. Physiol Rev. 2011;91(4):1447–531 Available from: http://www.ncbi.nlm.nih.gov/pubmed/22013216.
Mastroyiannopoulos NP, Nicolaou P, Anayasa M, Uney JB, Phylactou LA. Down-regulation of myogenin can reverse terminal muscle cell differentiation. PLoS One. 2012;7(1).
Bhatnagar S, Panguluri SK, Gupta SK, Dahiya S, Lundy RF, Kumar A. Tumor necrosis factor-α regulates distinct molecular pathways and gene networks in cultured skeletal muscle cells. PLoS One. 2010;5(10).
Sakuma K, Yamaguchi A. Sarcopenia and cachexia: the adaptations of negative regulators of skeletal muscle mass Cachexia Sarcopenia Muscle. 2012;3(2):77–94. https://doi.org/10.1007/s13539-011-0052-4.
López-Lluch G, Irusta PM, Navas P, de Cabo R. Mitochondrial biogenesis and healthy aging. Exp Gerontol. 2008;43:813–9.
Baker BM, Haynes CM. Mitochondrial protein quality control during biogenesis and aging. Trends Biochem Sci. 2011;36:254–61.
Braga M, Sinha Hikim AP, Datta S, Ferrini MG, Brown D, Kovacheva EL, et al. Involvement of oxidative stress and caspase 2-mediated intrinsic pathway signaling in age-related increase in muscle cell apoptosis in mice. Apoptosis. 2008;13(6):822–32.
Nitahara JA, Cheng W, Liu Y, Li B, Leri A, Li P, et al. Intracellular calcium, DNase activity and myocyte apoptosis in aging Fischer 344 rats. J Mol Cell Cardiol. 1998;30(3):519–35.
Cadenas E, Davies KJ. Mitochondrial free radical generation, oxidative stress, and aging. Free Radic Biol Med. 2000;29(3–4):222–30.
Dai D-F, Chiao YA, Marcinek DJ, Szeto HH, Rabinovitch PS. Mitochondrial oxidative stress in aging and healthspan. Longev Healthspan 2014;3(1):6. https://doi.org/10.1186/2046-2395-3-6.
Phillips T, Leeuwenburgh C. Muscle fiber specific apoptosis and TNF-alpha signaling in sarcopenia are attenuated by life-long calorie restriction. FASEB J. 2005;19(6):668–70 Available from: http://www.ncbi.nlm.nih.gov/pubmed/15665035.
Sakuma K, Aoi W, Yamaguchi A. Current understanding of sarcopenia: possible candidates modulating muscle mass. Pflugers Arch Eur J Physiol. 2014;467:213–29.
Chabi B, Ljubicic V, Menzies KJ, Huang JH, Saleem A, Hood DA. Mitochondrial function and apoptotic susceptibility in aging skeletal muscle. Aging Cell. 2008;7(1):2–12.
Calvani R, Joseph AM, Adhihetty PJ, Miccheli A, Bossola M, Leeuwenburgh C, et al. Mitochondrial pathways in sarcopenia of aging and disuse muscle atrophy. Biol Chem. 2013;394:393–414.
Bodine SC, Baehr LM. Skeletal muscle atrophy and the E3 ubiquitin ligases MuRF1 and MAFbx/atrogin-1. Am J Physiol Endocrinol Metab. 2014;307(6):E469–84.
Endo F, Tanaka Y, Tomoeda K, Tanoue A, Tsujimoto G, Nakamura K. Amino acids as regulators of proteolysis. Nutrition. 2003;133(4):2068–72.
Grumati P, Coletto L, Sabatelli P, Cescon M, Angelin A, Bertaggia E, et al. Autophagy is defective in collagen VI muscular dystrophies, and its reactivation rescues myofiber degeneration. Nat Med. 2010;16(11):1313–20.
Mariño G, Uría J, Puente X, Quesada V, Bordallo J, López-Otín C. Human autophagins, a family of cysteine proteinases potentially implicated in cell degradation by autophagy. J Biol Chem. 2003;278(6):3671–8.
Mammucari C, Milan G, Romanello V, Masiero E, Rudolf R, Del Piccolo P, et al. FoxO3 controls autophagy in skeletal muscle in vivo. Cell Metab. 2007;6(6):458–71.
O’Leary MFN, Hood DA. Denervation-induced oxidative stress and autophagy signaling in muscle. Autophagy. 2009;5(2):230–1.
Vainshtein A, Desjardins EM, Armani A, Sandri M, Hood DA. PGC-1α modulates denervation-induced mitophagy in skeletal muscle. Skelet Muscle. 2015:5–9 Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=4381453&tool=pmcentrez&rendertype=abstract.
Carter CS, Hofer T, Seo AY, Leeuwenburgh C. Molecular mechanisms of life- and health-span extension: role of calorie restriction and exercise intervention. Appl Physiol Nutr Metab. 2007;32(5):954–66.
Minor RK, Allard JS, Younts CM, Ward TM, De Cabo R. Dietary interventions to extend life span and health span based on calorie restriction. J Gerontol Ser A Biol Sci Med Sci. 2010;65A:695–703.
Roubenoff R. Catabolism of aging: is it an inflammatory process? Curr Opin Clin Nutr Metab Care [Internet]. 2003;6(3):295–9 Available from: http://content.wkhealth.com/linkback/openurl?sid=WKPTLP:landingpage&an=00075197-200305000-00005.
Sandri M. Signaling in muscle atrophy and hypertrophy. Physiology. 2008;23(3):160–70.
Ali S, Garcia JM. Sarcopenia, cachexia and aging: diagnosis, mechanisms and therapeutic options - a mini-review. Gerontology. 2014;60:294–305.
Wakimoto P, Block G. Dietary intake, dietary patterns, and changes with age: an epidemiological perspective. J Gerontol A Biol Sci Med Sci. 2001;56 Spec No(Ii):65–80.
Nieuwenhuizen WF, Weenen H, Rigby P, Hetherington MM. Older adults and patients in need of nutritional support: review of current treatment options and factors influencing nutritional intake. Clin Nutr. 2010;29:160–9.
Otsuka R, Kato Y, Nishita Y, Tange C, Tomida M, Nakamoto M, et al. Age-related changes in energy intake and weight in community-dwelling middle-aged and elderly Japanese. J Nutr Health Aging. 2016;20(4):383–90.
Landi F, Picca A, Calvani R, Marzetti E. Anorexia of aging. Clin Geriatr Med. 2017;33(3):315–23.
Maeda K, Takaki M, Akagi J. Decreased skeletal muscle mass and risk factors of sarcopenic dysphagia: a prospective observational cohort study. J Gerontol A Biol Sci Med Sci. 2017;72(9):1290–4.
Cederholm T, Barazzoni R, Austin P, Ballmer P, Biolo G, Bischoff SC, et al. ESPEN guidelines on definitions and terminology of clinical nutrition. Clin Nutr. 2017;36(1):49–64.
Muscaritoli M, Anker SD, Argilés J, Aversa Z, Bauer JM, Biolo G, et al. Consensus definition of sarcopenia, cachexia and pre-cachexia: Joint document elaborated by Special Interest Groups (SIG) “cachexia-anorexia in chronic wasting diseases” and “nutrition in geriatrics.”. Clin Nutr. 2010;29(2):154–9.
Robinson SM, Reginster JY, Rizzoli R, Shaw SC, Kanis JA, Bautmans I, et al. Does nutrition play a role in the prevention and management of sarcopenia? Clin Nutr. 2017;
Zello GA. Dietary reference intakes for the macronutrients and energy: considerations for physical activity. Appl Physiol Nutr Metab. 2006;31(1):74–9.
Coelho-Júnior HJ, Milano-Teixeira L, Rodrigues B, Bacurau R, Marzetti E, Uchida M. Relative protein intake and physical function in older adults: a systematic review and meta-analysis of observational studies. Nutrients. 2018;10(9).
Wolfe RR. Regulation of muscle protein by amino acids. J Nutr. 2002;132(10):3219S–24S.
Laplante M, Sabatini DM. An emerging role of mTOR in lipid biosynthesis. Vol. 19, Curr Biol. 2009.
Cuthbertson D, Smith K, Babraj J, Leese G, Waddell T, Atherton P, et al. Anabolic signaling deficits underlie amino acid resistance of wasting, aging muscle. FASEB J. 2005;19(3):422–4.
Drummond MJ, Miyazaki M, Dreyer HC, Pennings B, Dhanani S, Volpi E, et al. Expression of growth-related genes in young and older human skeletal muscle following an acute stimulation of protein synthesis. J Appl Physiol. 2009;106(4):1403–11.
Baum JI, Kim IY, Wolfe RR. Protein consumption and the elderly: what is the optimal level of intake? Vol. 8, Nutrients. 2016.
Bukhari SSI, Phillips BE, Wilkinson DJ, Limb MC, Rankin D, Mitchell WK, et al. Intake of low-dose leucine-rich essential amino acids stimulates muscle anabolism equivalently to bolus whey protein in older women at rest and after 1. Am J Physiol Endocrinol Metab. 2015;308(12):E1056–65.
Johnson MA, Kimlin MG. Vitamin D, aging, and the 2005 dietary guidelines for Americans. Nutr Rev. 2006;64(9):410–21.
Visser M, Deeg DJH, Lips P. Low vitamin D and high parathyroid hormone levels as determinants of loss of muscle strength and muscle mass (sarcopenia): the longitudinal aging study Amsterdam. J Clin Endocrinol Metab. 2003;88(12):5766–72.
Scott D, Blizzard L, Fell J, Ding C, Winzenberg T, Jones G. A prospective study of the associations between 25-hydroxy-vitamin D, sarcopenia progression and physical activity in older adults. Clin Endocrinol. 2010;73(5):581–7.
Ceglia L, Harris SS. Vitamin D and its role in skeletal muscle. Calcif Tissue Int. 2013;92:151–62.
Beaudart C, Dawson A, Shaw SC, Harvey NC, Kanis JA, Binkley N, et al. Nutrition and physical activity in the prevention and treatment of sarcopenia: systematic review. Osteoporos Int. 2017;28(6):1817–33.
Bunout D, Barrera G, Leiva L, Gattas V, de la Maza MP, Avendaño M, et al. Effects of vitamin D supplementation and exercise training on physical performance in Chilean vitamin D deficient elderly subjects. Exp Gerontol. 2006;41(8):746–52.
Houston DK, Neiberg RH, Tooze JA, Hausman DB, Johnson MA, Cauley JA, et al. Low 25-hydroxyvitamin D predicts the onset of mobility limitation and disability in community-dwelling older adults: the health ABC study. J Gerontol A Biol Sci Med Sci. 2013;68(2):181–7.
van Dronkelaar C, van Velzen A, Abdelrazek M, van der Steen A, Weijs PJM, Tieland M. Minerals and sarcopenia; the role of calcium, iron, magnesium, phosphorus, potassium, selenium, sodium, and zinc on muscle mass, muscle strength, and physical performance in older adults: a systematic review. J Am Med Dir Assoc. 2017; Available from:. https://doi.org/10.1016/j.jamda.2017.05.026.
Maggio M, Lauretani F, Ceda GP. Sex hormones and sarcopenia in older persons. Curr Opin Clin Nutr Metab Care. 2013;16(1):3–13.
Dent E, Morley JE, Cruz-Jentoft AJ, Arai H, Kritchevsky SB, Guralnik J, et al. International clinical practice guidelines for sarcopenia (ICFSR): screening, diagnosis and management. J Nutr Health Aging. 2018;22(10):1148–61.
Lenk K, Schuler G, Adams V. Skeletal muscle wasting in cachexia and sarcopenia: molecular pathophysiology and impact of exercise training. J Cachexia Sarcopenia Muscle. 2010;1:9–21.
Phu S, Boersma D, Duque G. Exercise and sarcopenia. J Clin Densitom. 2015;18(4):488–92.
Cruz-Jentoft AJ, Landi F, Schneider SM, Zúñiga C, Arai H, Boirie Y, et al. Prevalence of and interventions for sarcopenia in ageing adults: a systematic review. Report of the International Sarcopenia Initiative (EWGSOP and IWGS). Age Ageing. 2014;43(6):748–59.
Acknowledgments
This article was translated by Vanina Monique TUCCI-VIEGAS, Ph.D., Biologist & Translator/Reviewer of Scientific, Medical, and Technical Texts. E-mail address: vanina.monique@gmail.com.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Bottoni, A., dos Anjos Garnes, S., Lasakosvitsch, F. et al. Sarcopenia: an overview and analysis of molecular mechanisms. Nutrire 44, 6 (2019). https://doi.org/10.1186/s41110-019-0097-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41110-019-0097-2