Abstract
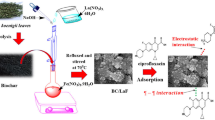
Due to the dual issues of antibiotic resistance and bioaccumulation toxicity, antibiotics are ubiquitously present in aquatic environments, and this is causing serious concern. Herein, novel nickel ferrite (NiFe2O4) nanoparticles were successfully loaded onto activated biochar (BC) derived from banana peel (BP) to obtain magnetic nanocomposite (BC-NiFe2O4) as an effective biosorbent for the ciprofloxacin antibiotic (CIP) elimination from pharmaceutical effluent. A facile co-precipitation approach was utilized to construct the heterogeneous BC-NiFe2O4. The synthesized materials were systematically characterized using techniques such as XRD, FE-SEM, EDX, HR-TEM, BET, FTIR, and XPS. In addition, the magnetic measurements indicated the ferromagnetic behavior of the BC-NiFe2O4 sample. The influencing factors (i.e., pH, contact time, initial concentration, dose of adsorbent, ions interference, and solution temperature) of the adsorption process were also well studied. The adsorption capacity of the BC-NiFe2O4 heterostructure was 68.79 mg g−1 compared to the BC sample (35.71 mg g−1), confirming that the loading of magnetically NiFe2O4 nanoparticles onto the surface of porous biochar enhanced its stability and adsorption performance for CIP removal, wherein the metal-antibiotic complex has a significant effect for the removal of CIP. Moreover, the Langmuir adsorption isotherm and the pseudo-second-order model displayed a good fit for the experimental data. The values of △H° and △G° revealed that the adsorption process was endothermic and spontaneous. The coordination affinities, π-π stacking, and H-bonding interactions play a more critical role in the adsorption mechanism that confirmed by FTIR and XPS analysis. To study the stability of BC-NiFe2O4 nanocomposites, desorption and recycling studies were investigated. The results revealed that after three cycles, no significant loss in removal efficiency was detected, reflecting the stability and reusability of the prepared BC-NiFe2O4 nanocomposite.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The emerging contaminants from pharmaceuticals are spread throughout all environmental matrices and therefore must be remediated before their discharge into chemical treatment plants (Ashiq et al. 2019; Aydin et al. 2019; Krasucka et al. 2021; Yao et al. 2021; Apreja et al. 2022; Okeke et al. 2022; Mittal et al. 2023). One of the medicinal products is the antibiotics family which are essential in the treatment of human and animal contagious diseases. Nonetheless, even at trace levels, there is widespread worry about their toxicity for non-target organisms (Li et al. 2019). One of the most widely used antibiotics for treating bacterial infections and stimulating animal growth is ciprofloxacin (CIP), a broad-spectrum antibiotic from the fluoroquinolone family that was classified by the World Health Organization (WHO) as a high-priority emerging organic pollutant (Egbedina et al. 2021). As a consequence of its widespread use, inadequate wastewater treatment and environmental discharge, CIP has been detected in a wide range of systems, including soil, sediment, drinking water, living tissues, and ground waters. This has sparked worries about their toxicity (Li et al. 2019; Dutta and Mala 2020). The existence of CIP in water ecosystems is particularly harmful to aquatic organisms (Li et al. 2019; Egbedina et al. 2021). The amount of ciprofloxacin measured in surface and groundwater is less than 1 g L−1 (Lins et al. 2020; Chaves et al. 2022). CIP concentrations in wastewater from healthcare and medication facilities may reach 150 g L−1 and 50 g L−1, respectively (Lins et al. 2020). Because of their high stability and wide dispersion, these antibiotics are non-dissolving long-range pollutants that must be removed before disposal. Fig. S1 depicts the life cycle of pollutants that emerge from healthcare facilities and pharmaceutical industries and diffuse to receptors. These antibiotics are highly detectable in water during their diffusion (Bhagat et al. 2020). To rapidly remove antibiotics from wastewater, efficient and commercially viable approaches should be developed. Reverse osmosis (Alonso et al. 2018), ion exchange (Wang et al. 2010, 2016; Bajpai and Bhowmik 2011), ultrafiltration (Palacio et al. 2018; Banerjee et al. 2019; Bhattacharya et al. 2019), ozonation (Gomes et al. 2017), advanced oxidation (Mondal et al. 2018), sedimentation, catalytic degradation (Gholami et al. 2020), and solvent extraction (Alaa El-Din et al. 2018; Akpomie and Conradie 2020) are some of the methods utilized to remove CIP from wastewater. The major disadvantages of these techniques include their high cost, hazardous by-product production, and difficult operability (Chen et al. 2021). Adsorption is a promising technique when compared to the prementioned methods because of its easy modification, reduced consumption, and low cost (Sun et al. 2019; Dai et al. 2020). Banana peel is a low-cost biosorbent with high adsorption behavior due to the number of adsorption active sites and natural components such as lignin and cellulose that are efficient in eliminating hazardous substances (Hashem et al. 2020). Biomass undergoes a variety of thermochemical processes, such as hydrothermal carbonization, and pyrolysis, to increase its adsorption capacity and kinetics (Wang et al. 2020). Biochar is produced from natural biomaterials by pyrolysis technique and has numerous applications in catalysis and water remediation due to its pore size and suitable physical and chemical properties (Lee et al. 2017). Biochar has received a lot of attention lately as a cheap and highly effective adsorbent due to its large pore volume, suitable physicochemical features, and ease of modification (Ouyang et al. 2020; Masrura et al. 2021). However, the low density of banana peel biochar implies insufficient dispersion in water. Consequently, the interaction ability of the soluble pollutants decreases, leading to a limited adsorption power. To overcome the separation difficulty, banana peel biochar is utilized as an accommodator for several magnetic nanoparticles. Due to their high adsorption effectiveness and ease of magnetic separation, magnetic nickel ferrite/biochar composites are a type of promising adsorbents; however, their synthesis involves with high costs and secondary environmental effects.
In this study, the highly magnetic NiFe2O4 nanoparticles were successfully imbedded on the surface of the biochar made from the banana peel biomass through a facile, rapid, and low cost co-precipitation method. The magnetic nanocomposite (BC-NiFe2O4) was used for the removal of ciprofloxacin antibiotic (CIP) from pharmaceutical wastewater. The metal antibiotics complex plays an important role in CIP removal. In addition, none of the available studies have utilized BC-NiFe2O4 heterogeneous structure for ciprofloxacin (CIP) removal and the sorption mechanisms of CIP were also not properly evaluated. Therefore, the interactions between bio-modified inorganic–organic waste and pharmaceutical sorption were investigated. Adsorption batch methodology was used to evaluate the influence of operational parameters on CIP removal, including solution pH, adsorbent dose, initial concentration of CIP, solution temperature, and interfering ions. The adsorption performance of porous biochar (BC) and BC-NiFe2O4 was examined using kinetic parameters, rate-controlling mechanisms, isotherms, and thermodynamics studies. Moreover, industrial pharmaceutical wastewater sample was studied. Potential adsorption mechanisms were proposed and verified using FTIR and XPS analyses.
Experimental
All materials and the characterization methodologies were listed in the electronic supplementary information (ESI).
Porous biochar preparation and its surface modification
Banana peel waste (BP) was used to produce activated biochar (BC) as previously indicated (Fig S2) (Azzam et al. 2022). In detail, banana peels (BPs) were washed with distilled water several times to remove any impurities and adhere contaminants. The cleaned banana peels were then chopped into 2*2 cm pieces and dried at 105 °C for 4.0 h until a constant weight was achieved (Munagapati et al. 2018; Chakhtouna et al. 2021). Using a laboratory sieve, the dried BPs were crushed to the proper size of 150 µm. To construct porous biochar (BC), banana peel powder was activated using H3PO4 by uniformly mixing 5 g of powdered BP with 10 mL of 30% H3PO4 for 30 min. Then, the mixture was calcined in air for two hours at 300 °C to carbonize the components of the mixture. The biochar was washed several times with distilled water to neutralize the solution's pH and eliminate any impurities. The produced biochar was then dried in an oven for 6 h at 80 °C.
Synthesis of biochar loaded NiFe 2 O 4 (BC-NiFe 2 O 4 )
Through a facile co-precipitation method, the highly magnetic NiFe2O4 nanoparticles were successfully imbedded on the surface of the biochar made from the banana peel biomass. Firstly, NiFe2O4 nanoparticles were prepared as follows, 0.1 g of nickel chloride and 0.57 g of ferric chloride were dissolved in 50 mL of distilled water for 30 min with continuous stirring at 50 °C. Sodium hydroxide (1.00 M) was added to the mixture to increase the pH above 12. Oleic acid was added to the mixture (1–2 drops) as a surfactant. After that, the mixture was stirred well for 30 min at 80 °C. The produced sample was washed with distilled water and ethanol to remove undesirable impurities and residual surfactant. The product was then centrifuged and dried overnight at 80 °C.
To construct BC-NiFe2O4 nanocomposites, 1.00 g of the obtained biochar was dispersed in 40 mL of deionized water for 10 min (solution A). The nickel and iron precursors were individually blended in 10 mL of deionized water, and the mixture was magnetically stirred well for 30 min at 50 °C (solution B). After carefully adding solution B to solution A, a drop of 1.00 M aqueous NaOH solution was added. The mixture was then heated for 45 min at 70 °C under magnetically stirring. After the reaction was completed, the mixture was centrifuged, washed with distilled water to neutralize the pH, and dried at 80 °C (Sagadevan et al. 2018; Chakhtouna et al. 2021).
Batch adsorption experiments
To determine the factors affecting the ability of the adsorbents to remove CIP, batch adsorption studies were conducted in 50-mL volumetric bottles at 25 °C. The following variables; pH (2.0–10.0), contact times (0–120 min), CIP concentrations (20–150 mg L−1), adsorbent dosage (5.0–100.0 mg), and the temperature (298–328 K) were investigated. The CIP concentration was directly measured at λmax 275 using a UV/Vis spectrophotometer (Mahmoud et al. 2021). The following equations were used to determine the adsorption performance qe (mg g−1) and CIP removal efficiency (%):
C0 represents the initial CIP concentration, Ce represents the CIP concentration at equilibrium (mg L−1), V represents the volume of the solution in liters, and W represents the weight of the adsorbate in grams (Avcı et al. 2019; Peñafiel et al. 2021).
Results and discussions
XRD analysis
The X-ray diffraction (XRD) patterns of BC and BC-NiFe2O4 nanocomposite were investigated as shown in Fig. 1. The BC diffraction pattern shows a characteristic a convex peak at 2θ ≈ 23° with a low dispersal angle, corresponding to graphitic-like microcrystals in biochar as identified by the JCPDS Card No. 46–1045 (Ding et al. 2014; Gupta and Gupta 2016; Tong et al. 2018; Patel et al. 2021a). This broad pattern suggests the amorphous properties of high porosity BC. In contrast, loading NiFe2O4 nanoparticles causes variations in biochar properties. The XRD pattern of the BC-NiFe2O4 sample exhibits distinct diffraction peaks at specific angles. These peaks are observed at 18.32° (111), 30.18° (220), 35.10° (311), 37.33° (222), 45.08° (400), 53.73° (422), 57.49° (511), and 63.13° (440), which are attributed to the presence of NiFe2O4 nanoparticles, as indicated by the JCPDS card No. 75–0035 (Hong et al. 2012; Livani et al. 2018; Sabaa et al. 2022). The crystallinity patterns of BC became more sharper after loading with NiFe2O4 nanoparticles. This findings implied that nickel ferrite (NiFe2O4) nanoparticles were successfully loaded onto porous biochar (BC).
Surface morphology
FE-SEM showed the structural morphology of the BC and BC-NiFe2O4 adsorbents. The biochar (BC) surface displayed multilayer porosity and dispersed particles with various shapes, as shown in (Fig. 2a, b). After NiFe2O4 loading, there are nano spherical particles deposited on the surface of BC sheets (Fig. 2c, d). According to the elemental mapping images of (Fig. 2e–i), the C, O, Ni, and Fe elements were distributed uniformly throughout the BC-NiFe2O4 sample. The BC-NiFe2O4 EDX spectrum, shown in Fig. 2j, demonstrates that spherical NiFe2O4 was successfully loaded onto the surface of BC.
HR-TEM was further conducted on the structure of the BC-NiFe2O4 nanocomposite. As shown in Fig. 3a, b, the BC-NiFe2O4 heterostructure has a clear irregular spherical structure with a diameter of 5–30 nm. The existence of lattice patterns (d-space = 0.180 nm) with lattice plane (220), related to NiFe2O4 nanoparticles (Fig. 3b), proves that the combination of BC and NiFe2O4 was successful. The appearance of bright points stacked on a ring pattern revealed by SAED confirms the polycrystallinity of BC-NiFe2O4 nanocomposites (Fig. 3c).
BET surface area and adsorption–desorption isotherm
The porosity characteristics of BC and BC-NiFe2O4 were investigated by studying the N2 adsorption–desorption isotherms and BET surface. The N2 isotherms of BC and BC-NiFe2O4 was illustrated in Fig. S4. According to IUPAC, type IV isotherms in the range of 0.5–1.0 P/P0 with obvious H3 hysteresis loops can be observed, indicating the formation of plentiful mesoporous structure (Sun et al. 2023).
As shown in Table 1, the specific surface area of BC (732 m2 g−1) was dramatically decreased after the loading of NiFe2O4 nanoparticles (252 m2 g−1) because of the blockage of BC pores with NiFe2O4 nanoparticles, confirming the successful formation of BC-NiFe2O4 heterostructures. Also, the total pore volume (VT) reduced from 0.553 to 0.201 cm3 g−1. Therefore, the external surface of BC became rougher and more heterogeneous after NiFe2O4 nanoparticles were loaded. In addition, the average pore size (DA) of BC and BC-NiFe2O4 samples was at 1.51 and 1.59 nm, respectively, showing that the two adsorbents are porous materials.
Magnetic properties
The magnetic properties of NiFe2O4 and BC-NiFe2O4 nanocomposite were investigated, and the results are presented in Table S1. The magnetic hysteresis curve (M-H) of the BC-NiFe2O4 nanocomposite was recorded at a temperature of zero Kelvin (0 K), as depicted in Fig. S5. The M-H plot displayed a characteristic hysteresis loop, indicating the ferromagnetic behavior of the BC-NiFe2O4 sample. This facilitates their recovery by using an external magnetic field. On the magnetic hysteresis curve, a saturation magnetization value of 10.02 emu g−1 was observed for the BC-NiFe2O4 nanocomposite which lower than pure NiFe2O4 nanoparticles (32.56 emu g−1). This decrease can be attributed to the presence of the banana peel biochar. It is worth noting that the majority of the composite material consists of banana peel biochar (BC). Consequently, the total magnetization may have been affected by the biochar’s nanomagnetic characteristics, which would indicate a lower saturation magnetization value in the BC-NiFe2O4 nanocomposite.
Adsorption performance evaluation
Influence of solution pH
Ciprofloxacin adsorption is dependent on pH-based speciation. The results showed the superiority of the CIP adsorption onto BC and BC-NiFe2O4 at pH 6.0. This finding could be explained by the CIP (pKa1 = 6.09, pKa2 = 8.64), meaning that it exists as protonated (pH < 6.09), deprotonated (pH > 8.64), neutral and zwitterionic forms (6.09 > pH < 8.64) (Patel et al. 2021b). Both zwitterionic and neutral ciprofloxacin occur between 6.09 and 8.64, with the zwitterion dominant. Its piperazine secondary aliphatic amine form is protonated at pH 6.09. The secondary amine of the zwitterion’s piperazine group or the carboxyl group of the neutral form deprotonate at a solution pH > 8.64 to produce anionic ciprofloxacin. The maximum removal ratio was at pH 6.0, where the CIP is predominantly as neutral and zwitterionic ciprofloxacin forms. All of these forms have the ability for electron donor–acceptor interaction, π-π stacking, and H-bonding. Further, BC and BC-NiFe2O4 have a point of zero charge (pHpzc) of 6.12 and 7.23, respectively. At pH > pHpzc, the removal % declined slightly due to charge-charge repulsions with negatively charged of adsorbents. When the pH < pHpzc, the CIP removal reduced due to a repulsive interaction with positively charged of adsorbents.
Influence of contact time and initial concentration
Figure 4b depicts the quantity of CIP adsorbed as a function of time. The removal efficiency of CIP onto BC and BC-NiFe2O4 samples was initially faster due to the presences of more available active sites on the surfaces of adsorbents. Almost 79.9% and 88.24% of the CIP were removed using BC and BC-NiFe2O4 within the first 10 min, respectively. Moreover, the adsorption percentages increased gradually until they reached equilibrium on BC and BC-NiFe2O4 after 90 min. Accelerated kinetics in the first 10 min are considered to be responsible for the increase of available active sites on the surface BC and BC-NiFe2O4 which gradually decreased over time due to the accumulation of CIP molecules on their surfaces reducing the uptake percentage (Tran et al. 2019). This phenomenon was caused by the general ionic migration of CIP into active pores and binding sites, which continued till all sites were filled (Yan et al. 2021).
Effect of a initial pH, b contact time, c initial CIP concentration, d adsorbent dosage, e solution temperature, and f interfering ions of CIP adsorption onto BC and BC-NiFe2O4 nanocomposite. ([CIP] = 40 mg L−1 (except a, c) (a); [CIP] = 20–150 mg L−1 (c); [adsorbent dosage] = 2 g L−1 (except d); T = 298 K (except e); without pH adjustment (except a))
On the other side, the initial CIP concentration has a significant influence on the adsorption performance of BC and BC-NiFe2O4 adsorbents. The impact of the initial CIP concentration was investigated between 20 and 150 mg L−1 at conditions (100 mg adsorbent; pH is 6; temperature 25 °C), as shown in Fig. 4c). The adsorption percentage of BC and BC-NiFe2O4 samples reduced from 98.2 to 22.2% and from 99.1 to 80.8%, respectively, when the initial concentration of CIP was raised from 20 to 150 mg L−1. The active sites on the surface adsorbents became unavailable as CIP concentrations increased. Comparing these results to our previous study on BC-NiS, they exhibited greater removal percentages (Azzam et al. 2022).
Influence of the adsorbent dose
Figure 4d illustrates the effect adsorbent dosage of BC and BC-NiFe2O4 onto the removal efficiency %. The increment in the dose of adsorbent from 5 to 100 mg enhanced the removal efficiency from 27.92 to 99.31% for BC-NiFe2O4. This increments have explained by providing more adsorption sites onto the surface of adsorbent (Peng et al. 2015; Abd El-Monaem et al. 2023; Omer et al. 2023).In addition, loading of NiFe2O4 into BC increased removal % to 99.31% compared to BC (95.82%).
Influence of solution temperature
The impact of temperature on the adsorption behavior of CIP onto BC and BC-NiFe2O4 was investigated from 298 to 328 K. As shown in Fig. 4e, raising the temperature improved removal efficiency % of CIP. The availability of more adsorption sites and increasing in CIP ions motion toward adsorbents accounts for the observed rise in removal percentages upon increasing the temperature. Therefore, the penetration of CIP molecules into the adsorbent pores is improved by raising the temperature (Kumari et al. 2020; Eltaweil et al. 2023).
Effect of ions interference
The effect of interfering ions on the CIP adsorption onto BC-NiFe2O4 heterostructure was investigated in the presence of several cations and anions. The presence of cations, e.g., Na+, K+, Mg2+, and Ca2+, slightly effect on CIP adsorption onto BC-NiFe2O4 but anion species such as Cl−, HCO3−, NO3−, and SO42− declined the removal efficacy (%) of CIP from 98.4 to 75.2, 79.5, 83.5, and 81.3%, respectively. This inhibitory effect was ascribed to competition for available sites on the BC-NiFe2O4 surface.
Adsorption kinetics
Understanding the adsorption mechanism is greatly facilitated by studying adsorption kinetics. Four different kinetic models based on the results of experiments were conducted to investigate the impact of contact time on the adsorption process. These models are Pseudo-first-order, pseudo-second-order (Li et al. 2020), Elovich (Ngakou et al. 2019), and the intraparticle diffusion (Genç and Dogan 2015), Eqs. (3–6), respectively. In addition, the RMSE (Root Mean Square Error) and χ2 (mathematically error) are used to measure the correlation between experimental data and theoretical models as shown in Eqs. (7 and 8).
where qe (mg g−1) represents the quantity of CIP removed per adsorbent weight at equilibrium and qt (mg/g) represents the quantity of CIP adsorbed per adsorbent weight at time t; k1 (min−1) and k2 (g mg−1 min) represent the rate constant of pseudo-first-order and pseudo-second-order rate constant (Eqs. 3, 4), repectively; α is initial adsorption rate (mg/g min), β is desorption constant (g mg−1); Kdiff (mg g−1 min −0.5) is the intraparticle diffusion constant, and L (g mg−1) represents the layer thickness. Figure 5 and Table 2 display the plots of kinetic models and their nonlinear parameters. As shown in Table 2, the coefficient of determination R2 values estimated from the pseudo-first-order model (≤ 0.439) was considerably lower than those calculated from the pseudo-second-order model (0.916), respectively. Moreover, the χ2 and RMSE values estimated using the pseudo-second-order model are found to be the smallest. As a result, the pseudo-second-order model (Fig. 5a) was a better fit to predict the adsorption of CIP onto the two adsorbents, which has been suggested to be chemisorption due to the excellent goodness between actual and predicted values. The Elovich model (Fig. 6S) describes the kinetics of chemisorption on a heterogeneous surface adsorbent, and Eq. 5 is used to interpret the experimental result (Ngakou et al. 2019). In addition, Table 2 shows that the Elovich model fits the kinetic data well, indicating that the adsorption of CIP antibiotic onto BC and BC-NiFe2O4 is a heterogeneous diffusion process rather than a first-order reaction. In addition, the intra diffusion model (Fig. 5b) can provide detailed information regarding the mass transfer process of CIP during adsorption. Obviously, the L value calculated from the intra diffusion model is not equal to zero. As a result, one might deduce that CIP removal occurred through a different mechanism. As a result, intra-particle diffusion also fit to further elucidate the kinetics and rate-limiting step in the adsorption of CIP. The entire adsorption phenomenon comprises two major steps: (i) external diffusion, where the CIP transfers from the solution to the surface of adsorbent; (ii) intra-particle or pore diffusion, where CIP diffusion transfer from the external surface of the adsorbents to the inner pores. It seems that the straight line from the origin diverges, which indicates that there are additional rate-controlling steps besides intraparticle diffusion including metal-antibiotic complex.
Isotherm studies
The adsorption isotherm relates the equilibrium concentration of CIP molecules in the solution to the amount of CIP antibiotic that is adsorbed per unit weight. The Langmuir model (Fig. 5c) is well-known for assuming monolayer adsorption on adsorbents with a homogeneous dispersion of adsorption sites, whereas the Freundlich model (Fig. 7S) is better suited for characterizing heterogeneous surface adsorption (Heo et al. 2019; Chakhtouna et al. 2021; Hu et al. 2021). The Temkin isotherm (Fig. 7S) suggests that the heat of adsorption of all molecules decreases with the molecular coverage of the adsorbent surface and the adsorption process is homogeneously distributed. Dubinin-Radushkevich (D-R) isotherm (Fig. 7S) explains the free energies of the adsorption (Foo and Hameed 2012; Ashiq et al. 2019; Rahdar et al. 2019; Mahmoud et al. 2021).
Equations (9–12) represent the Langmuir, Freundlich, Temkin, and D-R isotherms, respectively (Bonilla-Petriciolet et al. 2017; Li et al. 2020; Rodrigues et al. 2020).
where qe (mg g−1) is the amount of adsorbed CIP molecules per adsorbent materials weight; Ce (mg L−1) represents the residual of CIP. The maximum quantity of removal is represented by qm (mg g−1); the Langmuir and Freundlich constants are denoted by KL (L/mg) and Kf (mg g−1), respectively; (1/n) represents the adsorption intensity factor. KT (L g−1) is the binding constant of Temkin; B (RT/bT) is the heat generated from adsorption (J mol−1); bT is Temkin constant; qD-R represents an isotherm saturation capacity; β is D-R isotherm constant; ε is the Polanyi potentials (J/mol); and Es mean free energy (KJ mol−1); real gas constant (8.314 J mol−1 K−1) and temperature of solutions are denoted by R and T(K), respectively. The R2, χ2, and RMSE values for the adsorption of CIP on the BC and BC-NiFe2O4 show that the adsorption process effectively matches the Langmuir model (Fig. 5c, Table 3). The adsorption capacity of the BC-NiFe2O4 heterostructure was 68.79 mg g−1 compared to the BC sample (35.71 mg g−1) and NiFe2O4 nanoparticles (26.67 mg g−1). Moreover, BC-NiFe2O4 exhibited an efficient adsorption capacity compared to our previous work (Azzam et al. 2022). The bT values of BC and BC-NiFe2O4 were 42.97 and 6.82 kJ mol−1, respectively, demonstrating the endothermic nature of the adsorption process (El-Shafey et al. 2012). In addition, the mean free energy of the D-R model for BC and BC-NiFe2O4 was 2.29 and 0.707 kJ mol−1, respectively, which is smaller than 8 kJ mol−1. This results suggests that a physical process has a critical role in the adsorption of CIP onto BC and BC-NiFe2O4.
Thermodynamic study
Thermodynamic studies are useful for predicting the adsorption mechanisms of contaminants onto adsorbent materials. Several distinct types of thermodynamic metrics, such as enthalpy change (△H°), Gibbs free energy change (△G°), and entropy change (△S°), were evaluated to investigate the adsorption nature and spontaneity of CIP. As seen in Fig. 6, thermodynamic data were evaluated to depict how temperature affected the adsorption process. The impact of temperature was investigated in the range of 298 to 328 K. Herein, CIP antibiotic (40 mg L−1) was dispersed into 2 g L−1 of the BC or BC-NiFe2O4 samples at different temperatures (298, 308, 318, and 328 K) to study the thermodynamics of CIP removal using Van’t Hoff equations (Eqs. (13–16)).
where Kc (corrected Langmuir constant) can be determined from the Langmuir constant KL as follows:
△H° and △S° values were derived using the slope and intercept of lnKc against 1/T (Fig. 6), and △G° was calculated by using Eq. (13). M (g/mol) is the molecular weight of the CIP, R (kJ mol−1 K−1) is the real gas constant (8.314 × 10−3), and T is the solution’s temperature (K). Table 4 displays the thermodynamic parametars of CIP removal onto the adsorbent samples. The standard Gibbs free energies “△G°” are negative values, suggesting that CIP adsorption was spontaneous and feasible (Emily Chelangat Ngeno et al. 2016). The adsorption process was more effective at higher temperatures due to the ΔG° value for CIP adsorption onto the surfaces of the two adsorbents decreased as temperature increased. Furthermore, the positive values of △H° indicate the endothermic character of the adsorption process. On the other hand, the positive △S° values suggested the increase in randomness at the solid–liquid interface.
Proposal interpretation of the CIP adsorption mechanism
FTIR analysis
FTIR analysis (Fig. 7) was utilized to elucidate the adsorption mechanism because it is efficient at examining the functional groups of the adsorbents. The changes in the functional groups before and after the CIP removal is believed to be a strong indicator of the adsorption of adsorbate on the surface of BC and BC-NiFe2O4. The peak at 3364 cm−1 was attributed to the O–H stretching vibrations and responsible for water sensitivity or hydrophilicity. The C = C stretching and the carbonyl group (C = O) in the benzene rings in the BC sample were attributed to the vibrational peaks at 1616 and 1700 cm−1, respectively. These results showed relatively stable aromatic or graphitic structures that are favorable for contaminants’ adsorption through π-π interactions (Nogueira et al. 2018; Hu et al. 2021). Moreover, the peaks at 1376 cm−1 show the existence of C–O stretching vibration in the carboxylate groups. Furthermore, the existence of C-O stretching in the alcohol functional groups is demonstrated by peaks at 1042 cm−1 that shift to 1070 cm−1 in BC-NiFe2O4 because of the formation of H-bonding. The FTIR spectra of BC-NiFe2O4 showed two distinct peaks at 575 and 420 cm−1 (Ni–O-Fe and Fe–O) (Livani and Ghorbani 2018; Livani et al. 2018; Taj et al. 2021), indicating that the NiFe2O4 was constructed on the surface of biochar, which shifted to 535 and 410 cm−1 after CIP adsorption because of the metal coordination reactions. These results, which are consistent with the XRD analysis, confirm the successful preparation of BC-NiFe2O4 adsorbent (Aliahmad et al. 2013; Gor and Dave 2020). The peak located at 1390 cm−1 is related to the stretching C = O of ketones and quinones groups (Chakhtouna et al. 2021). Moreover, the stretching vibration of the C = C band of the aromatic ring in the BC-NiFe2O4 sample was assigned at 1570 cm−1 to 1630 cm−1 (Patel et al. 2021a). Therefore, these results confirm that the coordination reaction and π-π stacking further supported the adsorption mechanism between CIP and BC-NiFe2O4.
XPS analysis
Figure 8a shows the BC-NiFe2O4 XPS spectrum before and after the CIP adsorption. The XPS spectrum (Fig. 8a) shows clearly the characteristic peaks of Ni, O, C, Fe, and N elements, no impurity peaks were detected. The XPS spectrum of Ni 2p showed peaks for Ni 2p3/2 and Ni 2p1/2 (Fig. 8b). The peaks in the spin-orbital Ni 2p3/2 that are assigned to Ni2+ and Ni3+ have binding energies of 856.2, 861.6, 865.1, and 874.9 eV. The binding energies of the satellite peaks, denoted by the prefix “sat,” are 867.7 eV and 878.1 eV, respectively (Hua et al. 2018; Iraqui et al. 2020). Furthermore, the Ni 2p3/2 peaks were shifted to lower binding energies of 856.0, 861.7, 864.9, and 874.7 eV, and the intensity of all peaks were reduced, demonstrating that metallic coordination may be formed with CIP (Fig. 9e). Figure 8c shows the O 1 s core level spectrum, which had peaks at 531.3, 533.1, and 534.2 eV (Li et al. 2018a; Wu et al. 2021). The peak at 531.3 eV is related to the -C = O and O-C = O groups, whereas the peak at 533.1 eV is attributed to C–O–C and C–OH groups. Finally, the peak at 534.2 eV is assigned to the C-O group (Hua et al. 2018; Li et al. 2018a; Feng et al. 2021). The peak found at 530.8 eV after CIP adsorption was assigned to the carbonyl group. The observed substantial decrease in peak intensities 531.3 and 534.2 eV can also be attributed to the hydrogen bonding interactions between the O-C = O, C–OH groups and CIP molecules (shown in Fig. 9a). The XPS spectrum of C1s at a binding energy of 284.8 eV (Fig. 8d) is related to the C–C = C, C–C, and C-H groups of organic compounds. These organic compounds are appropriate for binding the CIP through π-π reaction (Fig. 9d). Moreover, the peak at 285.1 eV is ascribed to the C-O groups (Chowdhury et al. 2021). The binding energy for C–OH or C≡N groups is 286.72 eV (Yan et al. 2021). After CIP adsorption on biochar/nickel ferrite nanocomposite, a new peak appeared at 287.91 eV ascribed to the carbonyl groups. The Fe 2p core-level XPS spectrum (Fig. 8e) exhibits two broad peaks at 711.7 eV and 724.8 eV, which are assigned to Fe 2p3/2 and Fe 2p1/2, respectively, with a satellite peak of Fe 2p3/2 at 718.1 and 730.3 eV. These peaks confirm the existence of Fe (III) in the prepared adsorbent (BC-NiFe2O4) (Singh Yadav et al. 2015; Hua et al. 2018). Whereas, CIP loaded BC-NiFe2O4 shifted iron to lower binding energies of 710.3 eV and 723.6 eV, indicating that a metal-antibiotics complex may be formed (Fig. 9e). The N 1 s core level spectra is shown in Fig. 8f, with three peaks at 398.35, 400.41, and 401.98 eV. These peaks are related to N≡C group, C-N–C group, and the N–O group, respectively. After CIP adsorption, a new peak at 402.2 eV appeared, which is assigned to the quaternary N atom. The intensity of all peaks was decreased due to either hydrogen bonding or electrostatic interactions between the C-O on the adsorbent surface and the NH2+ in the ionic species of the CIP (Fig. 9b–d). Therfore, biochar contained a number of additional functional groups, such as C–O–C, C = O, C-N, C = N, and C–OH which could form a hydrogen bond with the CIP structure (-NH-, -COOH, and -F) and increase the CIP removal. In addition, the removal of CIP by BC and BC-NiFe2O4 based adsorbents is not only dependent on the pore volume, and pore structure, but is also influenced by other parameters such as coordination affinities, π-π stacking, and H-bonding interactions that confirmed by FTIR and XPS analysis. Although, the total pore volume (VT) of BC and BC-NiFe2O4 reduced from 0.553 to 0.201 cm3 g−1, respectively, but the removal % was enhanced, confirming other key factors affecting the adsorption process. This finding indicated that coordination affinities, π-π stacking, and H-bonding interactions demonstrated the dominant adsorption mechanisms.
Possible CIP adsorption mechanisms. a Speciation of CIP, (b) H-bonding between CIP zwitterion and biochar’s functional groups, c H-bonding between CIP zwitterion and biochar’s carbonyl groups, d possible π-π interactions between CIP and biochar, and e complexation reaction between metal-biochar and CIP
Environmental application of BC-NiFe 2 O 4 for CIP removal
The efficiency of BC-NiFe2O4 heterostructure as a CIP adsorbent was evaluated using three water samples collected from Helwan City, Egypt, including Nile water, groundwater, and pharmaceutical wastewater. A 0.1 g of BC-NiFe2O4 was mixed and spiked with 50 mL of each water sample with the initial concentration of 10 mg L−1 CIP. The initial pH values of solutions were adjusted to pH 6.0. The removal percentages are shown in Table S2. The CIP removal efficiency for Nile, ground, and pharmaceutical wastewater were 62.12%, 60.98%, and 93.81%, respectively. However, when an aqueous solution sample was used, it was 99.33%. The decrease in removal percentage can be explained by the presence of interfering ions.
Evaluation of reusability and comparison of BC-NiFe 2 O 4 removal efficacy with different adsorbents
The reusability of BC-NiFe2O4 composites was evaluated using 0.1 M sodium hydroxide (NaOH) as a stripping agent. In a batch experiment, BC-NiFe2O4-loaded CIP molecules were agitated in 50 mL of 0.1 M sodium hydroxide (NaOH) for 30 min. After regeneration, the adsorbent was washed several times with distilled water to remove the excess NaOH and dried at 80 °C to use in the next cycle. The removal efficiency of CIP after three cycles remained at 83.79%, as shown in Fig. 10a. Furthermore, as illustrated in Fig. 10b, the XRD analysis before and after regeneration has the same pattern, confirming the stability of BC-NiFe2O4 nanocomposites. Furthermore, the maximal adsorption capacity for CIP onto BC-NiFe2O4 via the Langmuir adsorption model was found to be 68.79 mg g−1, which is higher than previously reported adsorbents in the literature (Table 5). Therefore, BC-NiFe2O4 has a potential candidate for commercial application from pharmaceutical wastewater.
Conclusions
A highly efficient and stable BC-NiFe2O4 nanocomposite having magnetic properties was successfully developed via a facile co-precipitation approach for the elimination of ciprofloxacin (CIP) from pharmaceutical wastewater. The loading of magnetically NiFe2O4 nanoparticles on the surface of porous biochar enhanced the stability and adsorption performance for CIP removal compared to our previous work on biochar decorated with NiS (BC-NiS). The characterization techniques proved that the desired adsorbents had been successfully synthesized. Significantly, BC-NiFe2O4 showed the greatest adsorption capacity (68.79 mg g−1) compared to BC (35.71 mg g−1). Moreover, the removal of CIP using BC-NiFe2O4 was improved up to 99.3% within 30 min without pH adjustment. The results indicated that the adsorption process fits the Langmuir isotherm model, pseudo-second-order kinetics, and its endothermic nature. The removal effecieny of BC-NiFe2O4 adsorbent decreased slightly after three consecutive cycles, confirming the chemical stability of heterogenous strurcure. The FTIR and XPS analysis proved that the π-π interaction, the hydrogen bond and coordination affinities were identified as the primary driving forces of the adsorption mechanism of BC-NiFe2O4. These results showed that loading NiFe2O4 nanoparticles onto porous biochar has the potential to be a low-cost adsorbent for water remediation due to its easy manufacturing process, high removal effectiveness, and recyclable nature.
Data availability
All data generated or analyzed during this study are included in this published article.
References
Abd El-Monaem EM, Eltaweil AS, El-Subruiti GM et al (2023) Adsorption of nitrophenol onto a novel Fe3O4-κ-carrageenan/MIL-125(Ti) composite: process optimization, isotherms, kinetics, and mechanism. Environ Sci Pollut Res 30:49301–49313
Afzal MZ, Sun XF, Liu J et al (2018) Enhancement of ciprofloxacin sorption on chitosan/biochar hydrogel beads. Sci Total Environ 639:560–569
Agboola OS, Bello OS (2020) Enhanced adsorption of ciprofloxacin from aqueous solutions using functionalized banana stalk. Biomass Convers Biorefinery. https://doi.org/10.1007/s13399-020-01038-9
Akpomie KG, Conradie J (2020) Banana peel as a biosorbent for the decontamination of water pollutants. A Review Environ Chem Lett 18:1085–1112
Alaa El-Din G, Amer AA, Malsh G, Hussein M (2018) Study on the use of banana peels for oil spill removal. Alexandria Eng J 57:2061–2068
Aliahmad M, Noori, et al (2013) Synthesis of nickel ferrite nanoparticles by co-precipitation chemical method. Int J Phys Sci 8:854–858
Alicanoglu P, Sponza DT (2017) Removal of ciprofloxacin antibiotic with nano graphene oxide magnetite composite: comparison of adsorption and photooxidation processes. Desalin Water Treat 63:293–307
Alonso JJS, El Kori N, Melián-Martel N, Del Río-Gamero B (2018) Removal of ciprofloxacin from seawater by reverse osmosis. J Environ Manage 217:337–345
Apreja M, Sharma A, Balda S et al (2022) Antibiotic residues in environment: antimicrobial resistance development, ecological risks, and bioremediation. Environ Sci Pollut Res 29:3355–3371
Ashiq A, Sarkar B, Adassooriya N et al (2019) Sorption process of municipal solid waste biochar-montmorillonite composite for ciprofloxacin removal in aqueous media. Chemosphere 236:124384
Avcı A, İnci İ, Baylan N (2019) A comparative adsorption study with various adsorbents for the removal of ciprofloxacin hydrochloride from water. Water Air Soil Pollut 230:230–250
Aydin S, Aydin ME, Ulvi A, Kilic H (2019) Antibiotics in hospital effluents: occurrence, contribution to urban wastewater, removal in a wastewater treatment plant, and environmental risk assessment. Environ Sci Pollut Res 26:544–558
Azzam AB, Tokhy YA, El Dars FM, Younes AA (2022) Construction of porous biochar decorated with NiS for the removal of ciprofloxacin antibiotic from pharmaceutical wastewaters. J Water Process Eng 49:103006
Bajpai SK, Bhowmik M (2011) Poly(acrylamide-co-itaconic acid) as a potential ion-exchange sorbent for effective removal of antibiotic drug-ciprofloxacin from aqueous solution. J Macromol Sci Part A Pure Appl Chem 48:108–118
Bajpai SK, Bajpai M, Rai N (2012) Sorptive removal of ciprofoxacin hydrochloride from simulated wastewater using sawdust: kinetic study and effect of pH. Water SA 38:673–682
Banerjee S, Roy A, Madhusudhan MS et al (2019) Structural insights of a cellobiose dehydrogenase enzyme from the basidiomycetes fungus Termitomyces clypeatus. Comput Biol Chem 82:65–73
Bhagat C, Kumar M, Tyagi VK, Mohapatra PK (2020) Proclivities for prevalence and treatment of antibiotics in the ambient water: a review. npj Clean Water 3:42
Bhattacharya P, Mukherjee D, Dey S et al (2019) Development and performance evaluation of a novel CuO/TiO2 ceramic ultrafiltration membrane for ciprofloxacin removal. Mater Chem Phys 229:106–116
Bonilla-Petriciolet A, Mendoza-Castillo DI, Reynel-Ávila HE (2017) Adsorption processes for water treatment and purification
Chakhtouna H, Benzeid H, Zari N et al (2021) Functional CoFe2O4 -modified biochar derived from banana pseudostem as an efficient adsorbent for the removal of amoxicillin from water Hanane. Sep Purif Technol 266:118592
Chaves MJS de, Kulzer J, Pujol de Lima P da R, et al (2022) Updated knowledge, partitioning and ecological risk of pharmaceuticals and personal care products in global aquatic environments. Environ Sci Process Impacts 24:1982–2008
Chen J, Ouyang J, Cai X et al (2021) Removal of ciprofloxacin from water by millimeter-sized sodium alginate/H3PO4 activated corncob-based biochar composite beads. Sep Purif Technol 276:119371
Chowdhury A, Kumari S, Khan AA et al (2021) Activated carbon loaded with Ni-Co-S nanoparticle for superior adsorption capacity of antibiotics and dye from wastewater: kinetics and isotherms. Colloids Surfaces A Physicochem Eng Asp 611:125868
Dai Y, Zhou L, Tang X et al (2020) Macroporous ion-imprinted chitosan foams for the selective biosorption of U(VI) from aqueous solution. Int J Biol Macromol 164:4155–4164
Das KK, Patnaik S, Mansingh S et al (2020a) Enhanced photocatalytic activities of polypyrrole sensitized zinc ferrite/graphitic carbon nitride n-n heterojunction towards ciprofloxacin degradation, hydrogen evolution and antibacterial studies. J Colloid Interface Sci 561:551–567
Das S, Barui A, Adak A (2020b) Montmorillonite impregnated electrospun cellulose acetate nanofiber sorptive membrane for ciprofloxacin removal from wastewater. J Water Process Eng 37:101497
Dhiman N, Sharma N (2019) Batch adsorption studies on the removal of ciprofloxacin hydrochloride from aqueous solution using ZnO nanoparticles and groundnut (Arachis hypogaea) shell powder: a comparison*. Indian Chem Eng 61:67–76
Ding W, Dong X, Ime IM et al (2014) Pyrolytic temperatures impact lead sorption mechanisms by bagasse biochars. Chemosphere 105:68–74
Dutta J, Mala AA (2020) Removal of antibiotic from the water environment by the adsorption technologies: a review. Water Sci Technol 82:401–426
Egbedina AO, Adebowale KO, Olu-Owolabi BI et al (2021) Green synthesis of ZnO coated hybrid biochar for the synchronous removal of ciprofloxacin and tetracycline in wastewater. RSC Adv 11:18483–18492
El-Shafey ESI, Al-Lawati H, Al-Sumri AS (2012) Ciprofloxacin adsorption from aqueous solution onto chemically prepared carbon from date palm leaflets. J Environ Sci (china) 24:1579–1586
Eltaweil AS, Ibrahim K, Abd El-Monaem EM et al (2023) Phosphate removal by Lanthanum-doped aminated graphene oxide@aminated chitosan microspheres: insights into the adsorption mechanism. J Clean Prod 385:135640
Feng D, Guo D, Zhang Y et al (2021) Functionalized construction of biochar with hierarchical pore structures and surface O-/N-containing groups for phenol adsorption. Chem Eng J 410:127707
Foo KY, Hameed BH (2012) Potential of jackfruit peel as precursor for activated carbon prepared by microwave induced NaOH activation. Bioresour Technol 112:143–150
Genç N, Dogan EC (2015) Adsorption kinetics of the antibiotic ciprofloxacin on bentonite, activated carbon, zeolite, and pumice. Desalin Water Treat 53:785–793
Gholami P, Khataee A, Soltani RDC et al (2020) Photocatalytic degradation of gemifloxacin antibiotic using Zn-Co-LDH@biochar nanocomposite. J Hazard Mater 382:121070
Gomes J, Costa R, Quinta-Ferreira RM, Martins RC (2017) Application of ozonation for pharmaceuticals and personal care products removal from water. Sci Total Environ 586:265–283
Gor AH, Dave PN (2020) Adsorptive abatement of ciprofloxacin using NiFe2O4 nanoparticles incorporated into G. ghatti-cl-P(AAm) nanocomposites hydrogel: isotherm, kinetic, and thermodynamic studies. Polym Bull 77:5589–5613
Gupta H, Gupta B (2016) Adsorption of polycyclic aromatic hydrocarbons on banana peel activated carbon. Desalin Water Treat 57:9498–9509
Hashem AH, Saied E, Hasanin MS (2020) Green and ecofriendly bio-removal of methylene blue dye from aqueous solution using biologically activated banana peel waste. Sustain Chem Pharm 18:100333
Hassan SA, Ali FJ (2014) Determination of kinetics, thermodynamics and equilibrium parameters of ciprofloxacin adsorption from aqueous solution onto wastes of spent black tea leaves and pomegranate peel. Int J Adv Sci Tech Res 2:237–253
Heo J, Yoon Y, Lee G et al (2019) Enhanced adsorption of bisphenol A and sulfamethoxazole by a novel magnetic CuZnFe2O4–biochar composite. Bioresour Technol 281:179–187
Hong D, Yamada Y, Nagatomi T et al (2012) Catalysis of nickel ferrite for photocatalytic water oxidation using [Ru(bpy)3]2+ and S2O82-. J Am Chem Soc 134:19572–19575
Hu ZT, Ding Y, Shao Y et al (2021) Banana peel biochar with nanoflake-assembled structure for cross contamination treatment in water: interaction behaviors between lead and tetracycline. Chem Eng J 420:129807
Hua M, Xu L, Cui F et al (2018) Hexamethylenetetramine-assisted hydrothermal synthesis of octahedral nickel ferrite oxide nanocrystallines with excellent supercapacitive performance. J Mater Sci 53:7621–7636
Huang L, Wang M, Shi C et al (2014) Adsorption of tetracycline and ciprofloxacin on activated carbon prepared from lignin with H3PO4 activation. Desalin Water Treat 52:2678–2687
Iraqui S, Kashyap SS, Rashid MH (2020) CoFe2O4 nanoparticles: an efficient and reusable catalyst for the selective oxidation of benzyl alcohol to benzaldehyde under mild conditions. Nanoscale Adv 2:5790–5802
Khoshnamvand N, Ahmadi S, Mostafapour FK (2017) Kinetic and isotherm studies on ciprofloxacin an adsorption using magnesium oxide nanopartices. J Appl Pharm Sci 7:79–83
Krasucka P, Pan B, Sik Ok Y et al (2021) Engineered biochar – a sustainable solution for the removal of antibiotics from water. Chem Eng J 405:126926
Kumari S, Khan AA, Chowdhury A et al (2020) Efficient and highly selective adsorption of cationic dyes and removal of ciprofloxacin antibiotic by surface modified nickel sulfide nanomaterials: kinetics, isotherm and adsorption mechanism. Colloids Surfaces A Physicochem Eng Asp 586:124264
Lee J, Kim KH, Kwon EE (2017) Biochar as a catalyst. Renew Sustain Energy Rev 77:70–79
Li X, Wang W, Dou J et al (2016) Dynamic adsorption of ciprofloxacin on carbon nanofibers: quantitative measurement by in situ fluorescence. J Water Process Eng 9:14–20
Li J, Yu G, Pan L et al (2018a) Study of ciprofloxacin removal by biochar obtained from used tea leaves. J Environ Sci (china) 73:20–30
Li R, Wang Z, Guo J et al (2018b) Enhanced adsorption of ciprofloxacin by KOH modified biochar derived from potato stems and leaves. Water Sci Technol 77:1127–1136
Li R, Wang Z, Zhao X et al (2018c) Magnetic biochar-based manganese oxide composite for enhanced fluoroquinolone antibiotic removal from water. Environ Sci Pollut Res 25:31136–31148
Li MF, Liu YG, Zeng GM, Liu N, Liu SB et al (2019) Graphene and graphene-based nanocomposites used for antibiotics removal in water treatment: a review. Chemosphere 226:360–380
Li J, Yu G, Pan L et al (2020) Ciprofloxacin adsorption by biochar derived from co-pyrolysis of sewage sludge and bamboo waste. Environ Sci Pollut Res 27:22806–22817
Lins PVS, Henrique DC, Ide AH et al (2020) Adsorption of a non-steroidal anti-inflammatory drug onto MgAl/LDH-activated carbon composite – experimental investigation and statistical physics modeling. Colloids Surfaces A Physicochem Eng Asp 586:124217
Livani MJ, Ghorbani M (2018) Fabrication of NiFe2O4 magnetic nanoparticles loaded on activated carbon as novel nanoadsorbent for Direct Red 31 and Direct Blue 78 adsorption. Environ Technol (united Kingdom) 39:2977–2993
Livani MJ, Ghorbani M, Mehdipour H (2018) Preparation of an activated carbon from hazelnut shells and its composites with magnetic NiFe2O4 nanoparticles. Xinxing Tan Cailiao/new Carbon Mater 33:578–586
Mahmoud ME, Saad SR, El-Ghanam AM, Mohamed RHA (2021) Developed magnetic Fe3O4–MoO3-AC nanocomposite for effective removal of ciprofloxacin from water. Mater Chem Phys 257:123454
Masrura SU, Dissanayake P, Sun Y et al (2021) Sustainable use of biochar for resource recovery and pharmaceutical removal from human urine: a critical review. Crit Rev Environ Sci Technol 51:3016–3048
Mittal H, Ivaturi A, Khanuja M (2023) MoSe2-modified ZIF-8 novel nanocomposite for photocatalytic remediation of textile dye and antibiotic-contaminated wastewater. Environ Sci Pollut Res 30:4151–4165
Mondal SK, Saha AK, Sinha A (2018) Removal of ciprofloxacin using modified advanced oxidation processes: kinetics, pathways and process optimization. J Clean Prod 171:1203–1214
Munagapati VS, Yarramuthi V, Kim Y et al (2018) Removal of anionic dyes (Reactive Black 5 and Congo Red) from aqueous solutions using Banana Peel Powder as an adsorbent. Ecotoxicol Environ Saf 148:601–607
Ngakou CS, Anagho GS, Ngomo HM (2019) Non-linear regression analysis for the adsorption kinetics and equilibrium isotherm of phenacetin onto activated carbons. Curr J Appl Sci Technol 36:1–18
Ngeno EC, Orata F, Baraza LD et al (2016) Adsorption of caffeine and ciprofloxacin onto pyrolitically derived water hyacinth biochar: isothermal, kinetic and thermodynamic studies. J Chem Chem Eng 10:185–194
Nogueira J, António M, Mikhalev SM et al (2018) Porous carrageenan-derived carbons for efficient ciprofloxacin removal from water. Nanomaterials 8:1004
Okeke ES, Chukwudozie KI, Nyaruaba R et al (2022) Antibiotic resistance in aquaculture and aquatic organisms: a review of current nanotechnology applications for sustainable management. Springer, Berlin Heidelberg
Omer AM, Elgarhy GS, El-Subruiti GM et al (2023) Construction of efficient Ni-FeLDH@MWCNT@cellulose acetate floatable microbeads for Cr(VI) removal: performance and mechanism. Carbohydr Polym 311:120771
Ouyang J, Zhou L, Liu Z et al (2020) Biomass-derived activated carbons for the removal of pharmaceutical mircopollutants from wastewater: a review. Sep Purif Technol 253:117536
Palacio DA, Rivas BL, Urbano BF (2018) Ultrafiltration membranes with three water-soluble polyelectrolyte copolymers to remove ciprofloxacin from aqueous systems. Chem Eng J 351:85–93
Patel M, Kumar R, Pittman CU, Mohan D (2021) Ciprofloxacin and acetaminophen sorption onto banana peel biochars: environmental and process parameter influences. Environ Res 201:111218
Peñafiel ME, Matesanz JM, Vanegas E et al (2021) Comparative adsorption of ciprofloxacin on sugarcane bagasse from Ecuador and on commercial powdered activated carbon. Sci Total Environ 750:141498
Peng X, Hu F, Lam FLY et al (2015) Adsorption behavior and mechanisms of ciprofloxacin from aqueous solution by ordered mesoporous carbon and bamboo-based carbon. J Colloid Interface Sci 460:349–360
Rahdar A, Rahdar S, Ahmadi S, Fu J (2019) Adsorption of ciprofloxacin from aqueous environment by using synthesized nanoceria. Ecol Chem Eng S 26:299–311
Rodrigues DLC, Machado FM, Osório AG et al (2020) Adsorption of amoxicillin onto high surface area–activated carbons based on olive biomass: kinetic and equilibrium studies. Environ Sci Pollut Res 27:41394–41404
Sabaa HM, El-Khatib KM, El-Kady MY, Mahmoud SA (2022) Spinel structure of activated carbon supported MFe2O4 composites as an economic and efficient electrocatalyst for oxygen reduction reaction in neutral media. J Solid State Electrochem 26:2749–2763
Sagadevan S, Chowdhury ZZ, Rafique RF (2018) Preparation and characterization of nickel ferrite nanoparticles via co-precipitation method. Mater Res 21:21–25
Shang JG, Kong XR, He LL et al (2016) Low-cost biochar derived from herbal residue: characterization and application for ciprofloxacin adsorption. Int J Environ Sci Technol 13:2449–2458
Singh Yadav R, Havlica J, Masilko J et al (2015) Effects of annealing temperature variation on the evolution of structural and magnetic properties of NiFe2O4 nanoparticles synthesized by starch-assisted sol-gel auto-combustion method. J Magn Magn Mater 394:439–447
Sun W, Li H, Li H et al (2019) Adsorption mechanisms of ibuprofen and naproxen to UiO-66 and UiO-66-NH2: batch experiment and DFT calculation. Chem Eng J 360:645–653
Sun G, Zhang J, Li X et al (2023) Self-assembled morphology-controlled hierarchical Fe3O4 @LDH for Cr(VI) removal. J Environ Chem Eng 11:110129
Taj MB, Alkahtani MDF, Raheel A et al (2021) Bioconjugate synthesis, phytochemical analysis, and optical activity of NiFe2O4 nanoparticles for the removal of ciprofloxacin and Congo red from water. Sci Rep 11:1–19
Tang Y, Guo H, Xiao L et al (2013) Synthesis of reduced graphene oxide/magnetite composites and investigation of their adsorption performance of fluoroquinolone antibiotics. Colloids Surfaces A Physicochem Eng Asp 424:74–80
Tong W, Liu Q, Ren S et al (2018) Effect of pyrolysis temperature on pine sawdust chars and their gasification reactivity mechanism with CO2. Asia-Pacific J Chem Eng 13:2256
Van TT, Nguyen DTC, Le HTN et al (2019) MIL-53 (Fe)-directed synthesis of hierarchically mesoporous carbon and its utilization for ciprofloxacin antibiotic remediation. J Environ Chem Eng 7:102881
Wang CJ, Li Z, Jiang WT et al (2010) Cation exchange interaction between antibiotic ciprofloxacin and montmorillonite. J Hazard Mater 183:309–314
Wang W, Cheng J, Jin J et al (2016) Effect of humic acid on ciprofloxacin removal by magnetic multifunctional resins. Sci Rep 6:30331
Wang S, Zhang H, Huang H et al (2020) Influence of temperature and residence time on characteristics of biochars derived from agricultural residues: a comprehensive evaluation. Process Saf Environ Prot 139:218–229
Wu Y, Zheng H, Li H et al (2021) Magnetic nickel cobalt sulfide/sodium dodecyl benzene sulfonate with excellent ciprofloxacin adsorption capacity and wide pH adaptability. Chem Eng J 426:127208
Yan Y, Wang W, Peng Y et al (2021) Heterogeneous NiS/NiSe/3D porous biochar for As removal from water by interface engineering-induced nickel lattice distortion. Sci Total Environ 776:145874
Yao S, Ye J, Yang Q et al (2021) Occurrence and removal of antibiotics, antibiotic resistance genes, and bacterial communities in hospital wastewater. Environ Sci Pollut Res 28:57321–57333
Zhao J, Liang G, Zhang X et al (2019) Coating magnetic biochar with humic acid for high efficient removal of fluoroquinolone antibiotics in water. Sci Total Environ 688:1205–1215
Acknowledgements
We acknowledge the financial support from Helwan University, the National Research Centre (NRC), and the Central Metallurgical Research and Development Institute (CMRDI).
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
ABA and AAY: conception and design of study. ABA and YAT: acquisition of data. ABA, YAT, and AAY: analysis and/or interpretation of data. ABA, YAT, and AAY: drafting the manuscript. ABA, YAT, FMED, and AAY: revising the manuscript critically for important intellectual content. ABA, YAT, FMED, and AAY: approval of the version of the manuscript to be published.
Corresponding author
Ethics declarations
Ethical approval
This work does not contain any investigations with human participants or animals performed by any of the authors.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Guilherme Luiz Dotto
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Azzam, A.B., Tokhy, Y.A., Dars, F.M.E. et al. Heterogeneous porous biochar-supported nano NiFe2O4 for efficient removal of hazardous antibiotic from pharmaceutical wastewater. Environ Sci Pollut Res 30, 119473–119490 (2023). https://doi.org/10.1007/s11356-023-30587-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-023-30587-5