Abstract
The removal of antibiotics by adsorption was considered to be a cheap and efficient treatment method. Herein, magnetic NiFe2O4/biochar (NFO/BC) composites were prepared by hydrothermal growth of nickel ferrite nanoparticles on sawdust derived biochar. It was found that NFO/BC composites had developed pore structure, rich oxygen-containing functional groups, and strong magnetism. The maximum adsorption capacity of the prepared magnetic composite for tetracycline (TC) was 420.41 mg g−1. The results of adsorption thermodynamics and adsorption kinetics showed that the adsorption process is a spontaneous process, which conforms to Langmuir model and pseudo second-order kinetic model. The influence experiment of pH and ionic strength confirmed that electrostatic interaction and hydrogen bonding are the key factors affecting the adsorption process. Additionally, pore filling and π-π interaction also existed in the adsorption process of tetracycline. This recyclable magnetic composite provides a feasible research idea for the efficient removal of antibiotics from water.
Graphical Abstract

Highlights
• NiFe2O4/biochar magnetic composites were synthesized by hydrothermal method for TC removal.
• The enriched pore structure and expanded pore size of the composite were conducive to adsorbing TC.
• NiFe2O4/biochar exhibited higher TC removal efficiency and better recyclability than pure biochar and NiFe2O4.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Recently, the excessive use of antibiotics in many fields has led to the accumulation of a large amount of antibiotics in the water environment. These emerging pollutants have had adverse effects on ecosystems and human health. Therefore, how to effectively eliminate antibiotics in wastewater is an important research topic. So far, researchers have developed a series of methods, such as adsorption, photocatalysis, biodegradation, chemical oxidation, etc. (Wang et al. 2022). Among them, the adsorption method is simple and convenient to operate, low in energy consumption, high in efficiency, and does not produce secondary pollutants.
The preparation of adsorbent is the core task of adsorption method. High porosity materials, such as metal-organic-frameworks, covalent-organic-frameworks, and aerogels, have excellent adsorption capacity, but their complex synthesis process, high cost, and potential structural instability limit their use. Biochar was often used as adsorbent because of its large specific surface area, rich sources, low cost and other advantages (Wang et al. 2023). By modifying with acid and alkali, the surface oxygen-containing functional groups and porosity of biochar could be enhanced, resulting in improved adsorption performance (Jing et al. 2014; Sheng et al. 2022). However, traditional biochar has poor recyclability, which limits their practical application. The introduction of magnetic materials can easily separate biochar based adsorbents from water by using external magnetic field (Chen et al. 2017; Ma et al. 2022).
Ferrite is a general term for a class of composite oxides mainly composed of trivalent iron, with the general formula MFe2O4 (M refers to other divalent metals), which has strong stability and low saturation magnetic moment (Fang et al. 2021). MgFe2O4/biochar composite was prepared through co-precipitation and calcination method (Yao et al. 2021) using grapefruit peel as raw material. The results showed that the carbon matrix forms more edge sites with increasing pyrolysis temperature, which may contribute to the adsorption of (levofloxacin) LFX. The adsorption isotherm was well fitted by pseudo-second-order model, and the theoretical maximum adsorption capacity of MgFe2O4/biochar for LFX was 115 mg g−1. In addition, the superparamagnetic characteristic of MgFe2O4/biochar (66.815 emu g−1) is conducive to making it magnetically separated from water. CuCoFe2O4@Ch composite derived from chitosan was synthesized by a microwave method for TC adsorption (Nasiri et al. 2022). Under the optimal experimental conditions, the removal efficiency of TC was 93.07%, which was about 17% higher than that of pure CuCoFe2O4. The author suggested that the introduction of chitosan is beneficial to the dispersion of CuCoFe2O4 nanoparticles, leading to an increase in the adsorption area. In addition, the rich functional groups of chitosan, such as hydroxyl (OH), amine (NH2), etc., also help to strengthen the electrostatic force between the CuCoFe2O4@Ch and TC molecules. After four adsorption-desorption cycles, and the removal rate of TC by CuCoFe2O4@Ch could still reach up to 82.16%. MnFe2O4/biochar was used as the adsorbent for fluoroquinolone antibiotics (Xiang et al. 2020). The authors found that the surface properties of the adsorbent are closely related to the adsorption performance. Although both pefloxacin (PEF) and ciprofloxacin (CIP) belong to the fluoroquinolone antibiotics, since PEF is more hydrophobic than CIP, PEF exhibted a faster adsorption rate and larger adsorption capacity on MnFe2O4/biochar compared to CIP. The combination of biochar and ferrite not only improves the dispersion of magnetic materials, but also integrates the high porosity of biochar (Peng et al. 2021). The magnetic composites of biochar and NiFe2O4 were rarely reported for removing antibiotics.
In this study, NiFe2O4/biochar magnetic composites were synthesized by a simple hydrothermal method using poplar sawdust as the raw material. The microstructure, surface functional groups, and chemical composition were characterized, and the effects of operating parameters (initial pH, reaction time, addition amount, etc.) on adsorption were investigated through a series of batch experiments. The adsorption kinetics, thermodynamics isotherms and related subjects and models were used to analyze the adsorption performance of TC on the materials, and reveal its adsorption mechanism and potential application value.
2 Experimental
2.1 Materials and chemicals
The white poplar sawdust was collected from the local wood processing plant, crushed and passed through a 200 mesh sieve. Nickel(II) nitrate hexahydrate (Ni(NO3)2·6H2O), iron(III) nitrate nonahydrate (Fe(NO3)3·9H2O), and concentrated ammonia (NH3·H2O) were purchased from Sinopharm Chemical Reagent Co., Ltd. All reagents in the present study were of analytical grade and used without further purification. Tetracycline (98%) was purchased from Mackin Reagent Co., Ltd.
2.2 Preparation of the adsorbents
First, sawdust was cleaned with deionized water, dried and heated in a muffle furnace at 700 °C for 1 h. After cooling to room temperature, the resulting product was washed with deionized water and anhydrous ethanol to obtain biochar (BC).
NiFe2O4/biochar powder was synthesized with a hydrothermal method. The obtained BC (0.1 g) was dispersed into 50 mL of deionized water under sonication for 30 min. Then, 0.1 mmol Ni(NO3)2·6H2O and 0.2 mmol Fe(NO3)3·9H2O were slowly added into the above solution. Subsequently, a certain amount of concentrated ammonia solution was added dropwise until the pH value of the solution was adjusted to ~ 11. Finally, the above suspension was transferred to a 100 mL PTFE-lined autoclave and then heated at 180 °C for 12 h. The obtained precipitate was collected after centrifugation and washed with deionized water and anhydrous ethanol at least three times. The obtained product was dried in a vacuum oven at 80 °C for 12 h, named as NFO/BC. For comparison, NiFe2O4 powder was prepared under the same conditions without BC, labeled as NFO.
2.3 Physical characterizations
The porosity structures were investigated by N2-isothermal adsorption and desorption (Micromeritics ASAP 2460, USA), and the specific surface area and pore size distribution were calculated with Brunauer-Emmett-Teller (BET) method and Barrett-Joyner-Helenda (BJH) method, respectively. The vibrating sample magnetometer (VSM, Quantum Design PPMS, USA) was conducted to measure the magnetic properties of as-prepared samples at room temperature, and the test range of magnetization hysteresis curve was −30 ~ 30 KOe. The composition and crystal structure of the sample were determined by X-ray diffraction (XRD, Bruker D8-Advance, Germany) using Cu-Kα radiation source (λ = 0.15418 nm) at 40 kV and 30 mA. The scanning range and scanning rate were 20–80 o and 10 o min−1, respectively. Field emission scanning electron microscopy (SEM, Zeiss Supra-TM55, Germany) was carried out to observe the microstructure and morphology, meanwhile, the element distribution was recorded by energy dispersive X-ray spectroscopy (EDX). The surface functional groups of the samples were characterized by Fourier transform infrared spectroscopy (FTIR, Bruker VEC-TORTM 22, Germany) with a wavelength range of 4000 ~ 400 cm−1. X-ray photoelectron spectroscopy (XPS, Thermo ESCALAB 250Xi, USA) was used to characterize the samples’ surface chemical state, and the binding energy of all elements was calibrated by C 1 s (284.8 eV). Thermogravimetric analysis (TGA) was carried out on a TG analyzer (SDT Q600, TA Instruments, USA) at a heat rate of 10 °C min−1 between 25 °C and 800 °C in air.
2.4 Adsorption experiments
TC aqueous solution (20 mg L−1) was used to simulate wastewater. Before testing, the stock solution was diluted to a desired concentration to form experimental solutions. The pH value of each experimental solution was adjusted by 0.01 mol L−1 HCl and 0.01 mol L−1 NaOH solutions. All experimental solutions were shaken at 200 rpm min−1 in a dark box. The initial solution pH was adjusted by HCl (0.01 mol L−1) and NaOH (0.01 mol L−1) solutions. The adsorption isotherm experiments were carried out by vigorously shaking 20 mL of TC solution (5 ~ 200 mg L−1, pH = 6) mixed with 20 mg samples for 24 h to reach equilibrium. The adsorption kinetics was investigated by adding 10 mg of biochar into 20 mL TC solution (20 mg L−1, pH = 6). The supernatant was filtrated via centrifuging and the concentrations of TC were measured by UV-vis spectrophotometer (UV-5500PC, Metash, China) at the wavelength of 365.0 nm. The used adsorbent was washed to neutral by deionized water and ethanol and then dried at 60 °C for the cycle test. All the experiments were performed under identical conditions.
Adsorption capacity and removal rate were calcualated by the following Eqs. (1 and 2) (Kim and Yong 2021):
where, qe is the equilibrium adsorption capacity of the corresponding materials when the adsorption reaches equilibrium (mg g−1), R the removal rate (%), C0 the initial concentration of TC (mg L−1), Ce the concentration of TC when the adsorption reaches equilibrium (mg L−1), V the volume of the solution participating in the adsorption reaction (L), and m the mass of adsorbent (g).
3 Results and discussion
3.1 Physical and chemical properties of the as-prepared samples
The morphologies of as-prepared samples were observed by SEM. As illustrated from Fig. 1a, the NiFe2O4 nanoparticles were presented as an irregular sphere-like morphology with the diameters in the range of 10 ~ 20 nm. The typical tubular porous structures of BC were clearly observed in Fig. 1b, which derived from the natural structural characteristics of wood (Shan et al. 2021; Yu et al. 2020). It can be observed from Fig. 1c that the aggregate of nanoparticles looked more fluffy than that of pure NFO and the NFO nanoparticles deposited on BC (Fig. 1d).
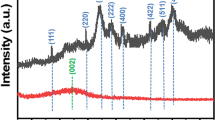
XRD was conducted to characterize the material composition and crystal structure of the synthesized sample, as shown in Fig. 2a. For NFO, the characteristic peaks appearing at 18.6 °, 30.3 °, 35.7 °, 43.1 °, 54.1 °, and 62.8 ° are consistent with the standard cards of trevorite NiFe2O4 (JCPDS 10–0325), corresponding to (111), (220), (311), (400), (422), (511), and (440) crystal planes, respectively (Rafique et al. 2016). In addition, no other impurity peaks were observed, indicating the successful preparation of pure NiFe2O4. Clearly, NFO/BC inherits all the characteristic peaks of NiFe2O4 and a broad peak of 25 ° originating from the diffraction peak derived from biochar, which indicates that NiFe2O4 was successfully loaded onto biochar.
N2 adsorption-desorption isotherms were used to study the specific surface area and pore pore distribution properties of the as-prepared samples, as shown in Fig. 2b. It is clear that all these three samples exhibited a type IV isotherm and type H4 hysteresis loop according to the International Union of Pure and Applied Chemistry (IUPAC) classification, proving the presence of mesopores structure (Qu et al. 2021). The difference is that the N2 adsorption capacity of BC and NFO/BC violently rised in the low relative pressure range (0 ≤ P/P0 ≤ 0.05), indicating that these two materials contain abundant micropores. The P/P0 range of hysteresis loop of NFO/BC was broader than NFO, implying that more pores were formed due to particle accumulation due to the loading of NFO after BC. The pore structural properties of these three samples are listed in Table 1. Obviously, due to the porous nature of wood, biochar had the highest BET specific surface area (422.5 m2 g−1) and pore volume (0.33 cm3 g−1), making it a good carrier for the growth of nanoparticles. Compared with BC, although the growth of NFO nanoparticles will cause the specific surface area of NFO/BC to decrease slightly (356.1 m2 g−1), its specific surface area was still 3 times that of pure NFO (111.6 m2 g−1), indicating that the BC carrier is beneficial to the dispersion of NFO. The expanded specific surface area of NFO/BC compared to NFO is beneficial for increasing the adsorption sites of TC, while the larger pore size of NFO/BC is more conducive to adsorbing TC than BC (Li et al. 2019). Additionally, the magnetism of the NFO/BC composite is in favour of the recovery of the adsorbent, in contrast to biochar.
The FT-IR spectra of the three samples are shown in Fig. 2c. The two absorption bands at 400 cm−1 ~ 600 cm−1 of NFO and NFO/BC belong to the tensile vibrations of Fe-O and Ni-O (Qin et al. 2023). The weak peaks at 869 cm−1 and 1124 cm−1 of BC and NFO/BC are attributed to the stretching vibration of C-O-C and bending vibration of C-H, respectively, and the peaks at 2850 cm−1 and 2925 cm−1 of BC and NFO/BC represent the stretching vibration of C-H bonds in biochar (You et al. 2021). Moreover, the bands near 1395 cm−1 and 1635 cm−1 of three samples can be indexed to the deformation vibration of adsorbed H2O, while the board peak at ~ 3400 cm−1 is related to the stretching vibration of -OH in the adsorbed H2O (Habibi and Parhizkar 2014). NFO/BC includes all of the characteristic groups of BC and NFO, and the presence of M-O groups also indicates the successful preparation of composite.
Magnetic separation is an important characteristic of recyclable adsorbents. The magnetic properties of NFO/BC composite samples at room temperature were studied by vibrating sample magnetometer. As shown in Fig. 2d, the field-dependent magnetization curve was not completely reversible, indicating the characteristic behavior of soft magnetic ferrites (Menelaou et al. 2014). The saturation magnetization (Ms), remanence magnetization (Mr) and coercivity (Hc) of NFO/BC are 15.2 emu g−1, 1.85 emu g−1 and 2.02 Oe, respectively, which is enough for easily separation of NFO/BC from sewage by a strong magnet.
Thermogravimetric analysis was used to determine the content of NiFe2O4 in the NFO/BC composites, as shown in Fig. S2. Clearly, the first stage of weightlessness occurs between 25 and 200 °C, which can be caused by the evaporation of adsorbed water. The second stage of weight loss lasts until 800 °C, mainly due to the pyrolysis and volatilization of cellulose, lignin, and other volatile substances, resulting in the most severe weight loss (Tsai and Liu 2016). As the temperature increases, the pyrolysis residue is mainly decomposed and carbonized, and the reaction rate is relatively slow, resulting in less than half of the remaining mass. It is speculated that the remaining material is mainly iron based oxides (Teh et al. 2021).
The surface composition and chemical state of the obtained samples were evaluated by XPS analysis (Fig. 3). The survey spectrum of the NFO/BC definitely proved the existence of C, O, Ni, and Fe in the composite (Fig. S3). For the high-resolution C 1 s spectra shown in Fig. 3a, three main peaks could be found at 284.8 eV, 285.9 eV, 287.1 eV, and 289.2 eV, attributing to the C=C/C-C, C=O, C-O, and π-π* vibration satellite peaks, respectively (Wu et al. 2020; Zhou et al. 2021a). The high-resolution O 1 s spectrum (Fig. 3b) is resolved into the peaks of metal-O (530.0 eV), C-O (531.3 eV) and -OH (532.1 eV), respectively (Wang et al. 2013). The Ni 2p spectrum (Fig. 3c) shows two typical peaks at 855.5 eV and 873.1 eV, corresponding to Ni 2p3/2 and Ni 2p1/2 respectively, along with its shake-up satellite peaks (861.4 eV and 879.8 eV) (Lin et al. 2022). The high-resolution Fe 2p spectrum (Fig. 3d) was fitted into five peaks, with peaks at 711.1 eV and 727.4 eV attributed to Fe3+ 2p3/2 and Fe2+ 2p1/2, respectively, while the peak at 724.4 eV belongs to the Fe3+ 2p1/2 orbital, also along with two satellite peaks at 715.1 eV and 719.3 eV (Zhou et al. 2021b).
3.2 Adsorption performance
First, the removal performance of three adsorbents on TC was compared, as shown in Fig. 4a. Under the same adsorption conditions, the removal rate of TC by NFO/BC can reach 93.9%, which is 1.9 times that of NFO and 1.67 times that of BC, respectively. Obviously, the composite adsorbent (NFO/BC) showed a higher removal ability for TC than the other two pure samples. As mentioned earlier, the NFO/BC composite has a higher specific surface area than pure NFO and more abundant mesopores than pure BC, thus synergistically providing the adsorption performance of the composite for TC. Subsequently, the effect of the adsorbent dose on the adsorption of TC was investigated, because the amount of adsorbent is closely related to the cost of wastewater treatment. The adsorbent dose was changed in the range of 0.2 ~ 2 .0 g L−1 at an initial TC concentration of 20.0 mg L−1. It was found that the removal rate of TC increased from 37% to 93.9% with the increasing of adsorbent dose from 0.2 to 1.0 g L−1, and tended to reach steady at the dose upto 2.0 g L−1 (Fig. 4a). It could be ascribed to the increased number of available adsorbent sites for TC adsorption with the increasing adsorbent dose (Bo et al. 2017). A slightly larger TC removal rate could be obtained when the amount of adsorbent increased to 2.0 g L−1, but the cost of adsorbent was doubled accordingly, which was obviously not cost-effective. Therefore, the adsorbent dose of 1.0 g L−1 was selected as the optimum adsorbent dose for further experiments.
The adsorption performance of different adsorbents for TC (a) and the effect of dosage on the adsorption performance (insert); zeta potential of NFO/BC at different pH (b); the effect of solution pH (c), humic acid (d) ion intensity (e) on removal rate of TC by NFO/BC and the reusability (f) of different adsorbents
The solution pH is a very important parameter which affects the surface charge of the adsorbent and the existence form of the TC, which in turn affects the adsorption behavior (Wang et al. 2015). First, the pH of zero charge point (pHpzc) of the as-prepared NFO/BC composite was tested by nanoparticle and zeta potential analyzer (Zetasizer Nano ZS90, Malvern, U.K.). As shown in Fig. 4b, the pHpzc of the NFO/BC was at ~ 5.35, indicating that the surface of NFO/BC was positively or negatively charged when the pH of the solution was below or above, respectively.
From Fig. 4c, it’s revealed that the TC removal efficiency is strongly dependent on the solution pH. Three dissociation constant (pKa) values of TC (pKa1 = 3.30, pKa2 = 7.68, and pKa3 = 9.69) correspond to three forms of cation state (TC+, pH < pK+a1), amphoteric or neutral state (TC±/TC0, pKa1 < pH < pKa2) and anion state (TC−, pH>pKa2) (Qiang and Adams 2004). When the solution pH value is 3 (less than the pHpzc of the adsorbent), the adsorbent will undergo protonation reaction, resulting in a positive charge on the NFO/BC surface (Huang et al. 2018). Hence, TC mainly exists in acidic solution (pH < 3.3) in the form of cation, which has electrostatic repulsion with the positive charge on the NFO/BC surface. In addition, there is a competitive adsorption relationship between H+ and TC cations in the water, resulting in a low removal rate of 75.5% at pH = 3.0. As the pH value further increased beyond the pHpzc of NFO/BC, the surface covert to negatively charged and can also electrostatic repel TC anions, leading to a decrease in adsorption capacity (Ai et al. 2020). The maximum removal percentage of TC was 92.4% at pH value of 7.0.
As an important component of dissolved organic matter, humic acid is widely present in sewage. Therefore, it is necessary to investigate the effect of humic acid on adsorption performance of NFO/BC. As shown in Fig. 4d, the removal rate of TC decreased significantly as the dosage of humic acid increased. The basic structure of humic acid is composed of aromatic rings and alicyclic rings, with abundant functional groups such as carboxyl, hydroxyl, carbonyl, quinone, and methoxy groups attached to the ring. Therefore, there may be strong competitive adsorption of humic acid and TC molecules on the complex (Niu et al. 2021).
The removal performance of pollutants is often influenced by the salts (especially inorganic salts) or competing ions (such as Na+, Ca2+, K+, Mg2+, Al3+, etc.) in the wastewater. Three types of metal ions (Na+, Mg2+, Ca2+) are widely present in groundwater. In this work, various inorganic salts (NaCl, MgCl2 or CaCl2) were introduced into simulated wastewater with equal concentration of TC to investigate the influence of metal ions on removal performance (Fig. 4e). Clearly, no matter how high a concentration of NaCl was added to the simulated wastewater, the adsorption behavior of TC was not significantly affected. In contrast, the introduction of MgCl2 and CaCl2 would lead to a significant decrease in the adsorption capacity of TC, indicating that Mg2+ and Ca2+ competed with TC to adsorb on the active site on the surface of NFO/BC. In addition, with the concentration of Mg2+ and Ca2+ increasing to 0.5%, the adsorption capacity of TC by NFO/BC gradually decreased to 59.8% and 63.8% compared with that in the original TC wastewater, respectively. This result illustrate that the high levels of competitive ions are more easily captured by adsorption sites. In addition, Mg2+ and Ca2+ ions are more likely to combine with water molecules, creating a hydration layer that hinders the contact between TC and the adsorbent (Fang et al. 2021).
For the large-scale applications, the recyclability of adsorbent is crucial for achieving cost-effective water treatment. In order to investigate the reusability, the TC adsorption and desorption of NFO/BC were performed for 4 cycles. Before each cycle, the adsorbent was filtered by centrifugation and desorbed by deionized water. Subsequently, the cleaned adsorbent was remixed with TC solution until adsorption saturation was reached again. As shown in Fig. 4f, the removal rate of TC by NFO/BC composite still maintained 72% after 4th cycle, indicating the good recyclability of NFO/BC. In addition, excellent magnetism also facilitates the easy separation of NFO/BC in large-scale water treatment processes.
3.3 Adsorption isotherms
Evaluating the adsorption isotherm is important for understanding the interaction between adsorbate and adsorbent. Langmuir and Freundlich isotherm adsorption models were commonly used to study the adsorption process (Liao et al. 2021). The Langmuir model is based on the assumption that the binding sites are uniformly distributed on the surface of the adsorbent. These binding sites have the same affinity for the adsorption of individual molecular layers. The adsorption energy is constant, and the adsorbate particles do not migrate on the surface plane. Compared with the Langmuir model, the Freundlich model assumes that stronger binding sites are first occupied, and the binding strength decreases with increasing site occupancy. It suggests that the adsorbent is covered by multiple layers of solute, and the adsorption on an energy non-uniform surface may have theoretical significance (Dai et al. 2020).
Freundlich isotherm model, as Eq. (3):
where, Qe, Ce, Kf, and n represent the equilibrium adsorption capacity (mg g−1), the concentration of adsorbate when the adsorption reaches equilibrium (mg L−1), the Freundlich affinity coefficient (mg1-n g−1 L-n), and the empirical parameter of adsorption strength.
Langmuir isotherm model, as Eq. (4):
where, Qm and KL denote as the maximum adsorption capacity (mg g−1), and the Langmuir adsorption constant (mg L−1).
The adsorption isotherm experiments were carried out by vigorously shaking 20 mL of TC solution (5 to 200 mg L−1) mixed with 20 mg adsorbent (BC, NFO, or NFO/BC) for 24 h to reach equilibrium. The experimental data were plotted and the fitting results are shown in Fig. 5. The isotherm constant and its correlation coefficient (R2) calculated from Langmuir and Freundlich models are listed in Table S1.
For the Langmuir isotherm model, the correlation coefficient of NFO (0.9923) and NFO/BC (0.9965) adsorption TC was much higher than that of BC (0.9856), indicating that the Langmuir isotherm has a better correlation with the actual experimental data of NFO and NFO/BC compared to BC. The magnetization effect may be the reason for the the formation of homogeneous monolayer adsorption sites (Zhao and Lang 2018). In addition, according to the Langmuir model, the enhanced maximum adsorption capacity of NFO/BC (420.4 mg g−1) for TC verified the stronger adsorption application ability of the composite.
The Freundich model was usually considered an empirical model for describing the adsorption of multiple molecular layers. The value of n in the Freundlich model is related to the properties of the adsorption material and the adsorption system. It was generally believed that pollutants could be easily adsorbed when n > 1. The larger n value corroborates the easier adsorption process. From Table S2, it can be seen that the 1/n values of these three samples were all less than 1, declaring that they may all adsorb antibiotics in a multi-molecular layer manner. Furthermore, the Kf value was related to the adsorption capacity of the adsorbents, and the larger the Kf value, the more favorable for adsorption. Obviously, the 1/n value of NFO/BC was the smallest and the Kf value of NFO/BC was the largest among these three samples, proving that TC is more inclined and maximizes non-uniform multi-layer adsorption on the surface of NFO/BC.
The adsorption performance of TC for different adsorbents with biochar or NiFe2O4 in the previous studies is listed in Table S2. It can be found that the NFO/BC composite was competitive.
3.4 Adsorption kinetics
Adsorption kinetics is an essential aspect for the operation to define the adsorption efficiency of a process. The adsorption kinetics were investigated by adding 20 mg of adsorbent into 20 mL TC solution (20 mg L−1). The obtained corresponding kinetic plots of the pseudo-first-order and pseudo-second-order models are shown in Fig. 6. It is observed that TC removal was very fast in the initial stage, and then became slow after increasing the contact time. In the early stage of adsorption, the initial concentration gradient of the solution was relatively high, and there were enough adsorption sites on the surface of the adsorbent. With the extension of adsorption time, the surface adsorption sites are occupied and TC molecules need to diffuse into the internal pores, resulting in a slower adsorption rate and ultimately reaching adsorption desorption equilibrium (Cheng et al. 2020). Clearly, under the same experimental conditions, the NFO/BC composite exhibited faster adsorption rate and higher adsorption capacity compared to pure NFO and BC, which was atrributed to the high specific surface area provided by biochar and more positive surface charges imparted by NFO nanoparticles.
The values of the obtained kinetic parameters and the correlation coefficients were calculated from the plots listed in Table S3. Compared with the pseudo-first-order model, the pseudo-second-order model showed higher correlation coefficient for the TC adsorption on the NFO/BC composite. Therefore, the TC adsorption process on NFO/BC was mainly dominated by chemisorption mechanism, including π-π electron donor-acceptor interactions (EDA), hydrogen bonding, and Metal-TC complexation (Fang et al. 2021; Zhang et al. 2023; Deng et al. 2022).
3.5 Adsorption thermodynamics
To investigate the impact of temperature on the adsorption processes, the following Eqs. (5–7) were used to calculate three thermodynamic parameters including the standard Gibbs free energy (∆G), enthalpy (∆H) and entropy (∆S):
where R (8.314 J mol−1 K−1) is the universal gas constant and T (K) is the absolute temperature, respectively.
The thermodynamic adsorption parameters at different temperatures are shown in Table S4. Through calculating the data at three different temperatures, it was found that all adsorbents had negative ∆G values, indicating that all of the TC adsorption processes were spontaneous. It is clear that the absolute value of ∆G of NFO/BC was much higher than that of the other two adsorbents at each temperature, implying the stronger adsorption driving force of TC by NFO/BC (Ndoun et al. 2021). In addition, for BC and NFO/BC, the absolute value of ∆G increased with increasing reaction temperature, while NFO showed the opposite trend. Therefore, it can be considered that the increase in reaction temperature could promote the spontaneous adsorption of TC by BC and NFO/BC, while the adsorption process of TC on NFO becomes more difficult. This viewpoint can also be confirmed by the vastly different ∆H value of NFO relative to BC and NFO/BC (Xia et al. 2019), because the values of enthalpy change (∆H) of BC and NFO/BC are positive, indicating that the TC adsorbing on these two substrates is endothermic. Hence, heating up is conducive to adsorption.
Moreover, the positive ∆S illustrates that the positive entropy change on the material surface during the adsorption process is greater than the negative entropy change caused by the reduction of tetracycline free energy, and the free energy at the solid-liquid interface increases. However, the adsorption of TC by NFO is an exothermic process, which indicates that its surface movement is restricted and the degree of disorder is reduced (Wang et al. 2018). Obviously, the ΔH value during the adsorption of TC molecules by the NFO/BC complex was 31.384 kJ mol−1 (> 20.9 kJ mol−1), indicating that the adsorption reaction was dominated by chemical adsorption, which is consistent with the adsorption kinetics results (Sun et al. 2020).
3.6 Adsorption mechanism
XRD, XPS, FTIR spectra, and N2 isothermal adsorption-desorption experiments were carried out to ascertain the change of BFO/BC before and after adsorption. As can be seen from Fig. 7a, the XRD diffraction peaks and peak positions of NFO/BC were changed after adsorption, indicating that the chemical properties of the adsorbate were stable in the solution environment. Figure 7b shows the high-resolution C1s XPS spectra of NFO/BC before and after adsorption of antibiotics. It can be seen that after the adsorption experiment, the peak area corresponding to C=C/C-C (at 284.8 eV) accounted for about 76.5%. Compared with before adsorption, the peak area increased by ~ 10%, which may be due to the adsorption of TC molecules on the NFO/BC surface, resulting in an increase in the atomic ratio of C=C/C-C (Deng et al. 2022; Yang Hc et al. 2021).
As presented in Fig. 7c, the characteristic peaks of NFO/BC after adsorption, at 1455 cm−1 and 1040 cm−1, could be ascribed to the the skeleton vibration of the benzene ring and the stretching vibration of C-OH in the tetracycline molecules, implying that the TC molecule was successfully adsorbed on NFO/BC. In addition, it can be seen that the two peaks of C-H and O=C-O almost disappeared after adsorption, attributing to the π-π EDA between NFO/BC and TC. Furthermore, the peak intensity corresponding to the -OH peak (~ 3400 cm−1) were weakened, indicating that the hydrogen bond interactions were involved in the adsorption process (Liu et al. 2019). Also, the weakening of M-O peak implies that M-O may provide part adsorption sites for the adsorption of TC.
Besides, the pore structure of the adsorbent also has a significant impact on the adsorption process. As shown in Fig. 7d, it is clear that NFO/BC before and after TC adsorption still exhibited similar adsorption isotherms (type IV), but the N2 adsorption volume of NFO/BC after adsorption experiment significantly decreased compared with that of the pristine sample, which is atrributed to the adsorbed TC molecules occupying the pores of the adsorbent through pore-filling mechanism. The huge change in the pore structure of the adsorbent before and after TC adsorption can also be more intuitively observed from Fig. S3 and Table S5. Obviously, the BET surface area and pore volume of NFO/BC was reduced to 174.6 m2 g−1 and 0.19 cm3 g−1 after TC adsorption, almost half of that of the pristine sample.
4 Conclusions
In conclusion, a magnetic composite (NFO/BC) was prepared by growth of nickel ferrite nanoparticles on biochar by hydrothermal method, which was used as the adsorbent for antibiotics in water. The introduction of NFO gives biochar good magnetic properties, and biochar helps improve the dispersion of NFO nanoparticles. Compared with pure BC and NFO, the TC removal rate of the NFO/BC composite increased by more than 1 times. In addition, the removal rate of TC by NFO/BC composite still maintained 72% after 4th cycle, indicating the good recyclability of NFO/BC. The experimental data are in good agreement with the pseudo-second-order kinetic model and Langmuir model. Thermodynamic parameters indicate that adsorption was endothermic and spontaneous. The adsorption process of TC on NFO/BC may be synergistically influenced by hydrogen bonding, π-π electron donor-acceptor interaction, electrostatic interactions, and pore filling. The efficient TC removal rate, fast adsorption kinetics, and good recyclability of the magnetic NFO/BC composite demonstrate its feasibility in the treatment of tetracycline-contaminated water.
Availability of data and materials
The datasets used or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- NFO/BC:
-
NiFe2O4/Biochar
- TC:
-
Tetracycline hydrochloride
- PTFE:
-
Polytetrafluoroethylene
References
Ai T, Jiang XJ, Liu QY, Lv LL, Dai SJ (2020) Single-component and competitive adsorption of tetracycline and Zn(ii) on an NH4Cl-induced magnetic ultra-fine buckwheat peel powder biochar from water: studies on the kinetics, isotherms, and mechanism. RSC Adv 10:20427–20437. https://doi.org/10.1016/10.1039/d0ra02346a
Bo X, Ling D, Lou H, Gu HB (2017) 3D hierarchical flower-like nickel ferrite/manganese dioxide toward lead (II) removal from aqueous water. J Hazard Mater 325:178–188. https://doi.org/10.1016/10.1016/j.jhazmat.2016.11.011
Chen SQ, Chen YL, Jiang H (2017) Slow pyrolysis magnetization of Hydrochar for effective and highly stable removal of tetracycline from aqueous solution. Ind Eng Chem Res 56:3059–3066. https://doi.org/10.1016/10.1021/acs.iecr.6b04683
Cheng DL, Ngo HH, Guo WS, Chang SW, Nguyen DD, Zhang XB, Varjani S, Liu Y (2020) Feasibility study on a new pomelo peel derived biochar for tetracycline antibiotics removal in swine wastewater. Sci Total Environ 720:137662. https://doi.org/10.1016/10.1016/j.scitotenv.2020.137662
Dai J, Meng X, Zhang Y, Huang Y (2020) Effects of modification and magnetization of rice straw derived biochar on adsorption of tetracycline from water. Bioresour Technol 311:123455. https://doi.org/10.1016/10.1016/j.biortech.2020.123455
Deng Y, Wang M, Yang YP, Li X, Chen WQ, Ao TQ (2022) Enhanced adsorption performance of sulfamethoxazole and tetracycline in aqueous solutions by MgFe2O4-magnetic biochar. Water Sci Technol 86:568–583. https://doi.org/10.1016/10.2166/wst.2022.227
Fang L, Miao YX, Wei D, Zhang Y, Zhou YC (2021) Efficient removal of norfloxacin in water using magnetic molecularly imprinted polymer. Chemosphere 262:128032. https://doi.org/10.1016/10.1016/j.chemosphere.2020.128032
Habibi MH, Parhizkar HJ (2014) FTIR and UV–vis diffuse reflectance spectroscopy studies of the wet chemical (WC) route synthesized nano-structure CoFe2O4 from CoCl2 and FeCl3. Spectrochim Acta A Mol Biomol Spectrosc 127:102–106. https://doi.org/10.1016/10.1016/j.saa.2014.02.090
Huang P, Ge CJ, Feng D, Yu HM, Luo JW, Li JT, Strong PJ, Sarmah AK, Bolan NS, Wang HL (2018) Effects of metal ions and pH on ofloxacin sorption to cassava residue-derived biochar. Sci Total Environ 616:1384–1391. https://doi.org/10.1016/10.1016/j.scitotenv.2017.10.177
Jing XR, Wang YY, Liu WJ, Wang YK, Jiang H (2014) Enhanced adsorption performance of tetracycline in aqueous solutions by methanol-modified biochar. Chem Eng J 248:168–174. https://doi.org/10.1016/10.1016/j.cej.2014.03.006
Kim D, Yong K (2021) Boron doping induced charge transfer switching of a C3N4/ZnO photocatalyst from Z-scheme to type II to enhance photocatalytic hydrogen production. Appl Catal B 282:119538. https://doi.org/10.1016/10.1016/j.apcatb.2020.119538
Li C, Zhu X, He H, Fang Y, Dong H, Lü J, Li J, Li Y (2019) Adsorption of two antibiotics on biochar prepared in air-containing atmosphere: influence of biochar porosity and molecular size of antibiotics. J Mol Liq 274:353–361. https://doi.org/10.1016/10.1016/j.molliq.2018.10.142
Liao Q, Rong H, Zhao M, Luo H, Chu Z, Wang R (2021) Interaction between tetracycline and microorganisms during wastewater treatment: a review. Sci Total Environ 757:143981. https://doi.org/10.1016/10.1016/j.scitotenv.2020.143981
Lin HT, Qiu SJ, Wu ZH, Ye XX, Liu M, Hua (2022) Fabrication of lignin-based biochar containing multi-metal ferrite and efficient removal for oxytetracycline hydrochloride, J Clean Prod 331:129885. https://doi.org/10.1016/10.1016/j.jclepro.2021.129885
Liu JL, Zhou BQ, Zhang H, Ma J, Mu B, Zhang WB (2019) A novel biochar modified by chitosan-Fe/S for tetracycline adsorption and studies on site energy distribution. Bioresour Technol 294:122152. https://doi.org/10.1016/10.1016/j.biortech.2019.122152
Ma YF, Lu TM, Yang L, Wu L, Li P, Tang JY, Chen YL, Gao F, Cui S, Qi XB, Zhang ZL (2022) Efficient adsorptive removal of fluoroquinolone antibiotics from water by alkali and bimetallic salts co-hydrothermally modified sludge biochar. Environ Pollut 298:118833. https://doi.org/10.1016/10.1016/j.envpol.2022.118833
Menelaou M, Georgoula K, Simeonidis K, Dendrinou-Samara C (2014) Evaluation of nickel ferrite nanoparticles coated with oleylamine by NMR relaxation measurements and magnetic hyperthermia. Dalton Trans 43:3626–3636. https://doi.org/10.1016/10.1039/C3DT52860J
Nasiri A, Rajabi S, Amiri A, Fattahizade M, Hasani O, Lalehzari A, Hashemi M (2022) Adsorption of tetracycline using CuCoFe2O4@chitosan as a new and green magnetic nanohybrid adsorbent from aqueous solutions: isotherm, kinetic and thermodynamic study. Arab J Chem 15:104014. https://doi.org/10.1016/10.1016/j.arabjc.2022.104014
Ndoun MC, Elliott HA, Preisendanz HE, Williams CF, Knopf A, Watson JE (2021) Adsorption of pharmaceuticals from aqueous solutions using biochar derived from cotton gin waste and guayule bagasse. Biochar 3:89–104. https://doi.org/10.1016/10.1007/s42773-020-00070-2
Niu HQ, Yang HY, Tong LL (2021) Adsorption behaviors of au(III) onto humic acid extracted from gold ore: adsorptive kinetics, isotherm and mechanism. Colloids Surf A Physicochem Eng Asp 630:127442. https://doi.org/10.1016/10.1016/j.colsurfa.2021.127442
Peng H, Li YX, Wen J, Zheng XG (2021) Synthesis of ZnFe2O4/B, N-codoped biochar via microwave-assisted pyrolysis for enhancing adsorption-photocatalytic elimination of tetracycline hydrochloride. Ind Crop Prod 172:114066. https://doi.org/10.1016/10.1016/j.indcrop.2021.114066
Qiang ZM, Adams C (2004) Potentiometric determination of acid dissociation constants (pKa) for human and veterinary antibiotics. Water Res 38:2874–2890. https://doi.org/10.1016/10.1016/j.watres.2004.03.017
Qin HD, Cheng H, Shi W, Wu SZ, Chen J, Huang JM, Li H (2023) Heterogeneous degradation of cefotaxime sodium with peroxymonosulfate activated by an effective and magnetically separable Ni0.5Zn0.5Fe2O4 catalyst. J Environ Chem Eng 11:109070. https://doi.org/10.1016/10.1016/j.jece.2022.109070
Qu JH, Wang YX, Tian X, Jiang Z, Deng FX, Tao Y, Jiang Q, Wang L, Zhang Y (2021) KOH-activated porous biochar with high specific surface area for adsorptive removal of chromium (VI) and naphthalene from water: affecting factors, mechanisms and reusability exploration. J Hazard Mater 401:123292. https://doi.org/10.1016/10.1016/j.jhazmat.2020.123292
Rafique MY, Ellahi M, Iqbal MZ, Q-u-a J, Pan LQ (2016) Gram scale synthesis of single crystalline nano-octahedron of NiFe2O4: magnetic and optical properties. Mater Lett 162:269–272. https://doi.org/10.1016/10.1016/j.matlet.2015.10.025
Shan XF, Wu J, Zhang XT, Wang L, Yang JL, Chen ZJ, Yu JF, Wang XM (2021) Wood for application in electrochemical energy storage devices. Cell Rep Phys Sci 2:100654. https://doi.org/10.1016/10.1016/j.xcrp.2021.100654
Sheng XY, Wang JK, Cui QT, Zhang W, Zhu XF (2022) A feasible biochar derived from biogas residue and its application in the efficient adsorption of tetracycline from an aqueous solution. Environ Res 207:112175. https://doi.org/10.1016/10.1016/j.envres.2021.112175
Sun YZ, Chen M, Liu H, Zhu Y, Wang DB, Yan M (2020) Adsorptive removal of dye and antibiotic from water with functionalized zirconium-based metal organic framework and graphene oxide composite nanomaterial Uio-66-(OH)2/GO. Appl Surf Sci 525:146614. https://doi.org/10.1016/10.1016/j.apsusc.2020.146614
Teh JS, Teoh YH, How HG, Sher F (2021) Thermal analysis Technologies for Biomass Feedstocks: a state-of-the-art review. Processes 9:1610. https://doi.org/10.1016/10.3390/pr9091610
Tsai WT, Liu SC (2016) Thermochemical characterization of cattle manure relevant to its energy conversion and environmental implications. Biomass Convers Biorefin 6:71–77. https://doi.org/10.1016/10.1007/s13399-015-0165-7
Wang H, Fang CR, Wang Q, Chu YX, Song YL, Chen YM, Xue XD (2018) Sorption of tetracycline on biochar derived from rice straw and swine manure. RSC Adv 8:16260–16268. https://doi.org/10.1016/10.1039/c8ra01454j
Wang K, Wang Y, Zhang SY, Chen YD, Wang RP, Ho SH (2022) Tailoring a novel hierarchical cheese-like porous biochar from algae residue to boost sulfathiazole removal. Environ Sci Ecotechnol 10:100168. https://doi.org/10.1016/10.1016/j.ese.2022.100168
Wang P, Du ML, Zhu H, Bao SY, Yang TT, Zou ML (2015) Structure regulation of silica nanotubes and their adsorption behaviors for heavy metal ions: pH effect, kinetics, isotherms and mechanism. J Hazard Mater 286:533–544. https://doi.org/10.1016/10.1016/j.jhazmat.2014.12.034
Wang RP, Zhang SY, Chen HL, He ZX, Cao GL, Wang K, Li FH, Ren NQ, Xing DF, Ho SH (2023) Enhancing biochar-based nonradical persulfate activation using data-driven techniques. Environ Sci Technol 57:4050–4059. https://doi.org/10.1016/10.1021/acs.est.2c07073
Wang T, Zhang LY, Wang HY, Yang WC, Fu YC, Zhou WL, Yu WT, Xiang KS, Su Z, Dai S, Chai LY (2013) Controllable synthesis of hierarchical porous Fe3O4 particles mediated by poly(diallyldimethylammonium chloride) and their application in arsenic removal. ACS Appl Mater Interfaces 5:12449–12459. https://doi.org/10.1016/10.1021/am403533v
Wu J, Wang YH, Wu ZX, Gao Y, Li XP (2020) Adsorption properties and mechanism of sepiolite modified by anionic and cationic surfactants on oxytetracycline from aqueous solutions. Sci Total Environ 708:134409. https://doi.org/10.1016/10.1016/j.scitotenv.2019.134409
Xia Y, Yang TX, Zhu NM, Li D, Chen ZL, Lang QQ, Liu Z, Jiao WT (2019) Enhanced adsorption of Pb(II) onto modified hydrochar: modeling and mechanism analysis. Bioresour Technol 288:121593. https://doi.org/10.1016/10.1016/j.biortech.2019.121593
Xiang YJ, Yang X, Xu ZY, Hu WY, Zhou YY, Wan ZH, Yang YH, Wei YY, Yang J, Tsang DCW (2020) Fabrication of sustainable manganese ferrite modified biochar from vinasse for enhanced adsorption of fluoroquinolone antibiotics: effects and mechanisms. Sci Total Environ 709:136079. https://doi.org/10.1016/10.1016/j.scitotenv.2019.136079
Yang Hc YH, Wang J, Ning T, Chen P, Yu J, Sy D, Sk Z (2021) Magnetic porous biochar as a renewable and highly effective adsorbent for the removal of tetracycline hydrochloride in water. Environ Sci Pollut Res 28:61513–61525. https://doi.org/10.1016/10.1007/s11356-021-15124-6
Yao B, Luo ZR, Du SZ, Yang J, Zhi D, Zhou YY (2021) Sustainable biochar/MgFe2O4 adsorbent for levofloxacin removal: adsorption performances and mechanisms. Bioresour Technol 340:125698. https://doi.org/10.1016/10.1016/j.biortech.2021.125698
You Y, Shi ZK, Li YH, Zhao ZJ, He B, Cheng XW (2021) Magnetic cobalt ferrite biochar composite as peroxymonosulfate activator for removal of lomefloxacin hydrochloride. Sep Purif Technol 272:118889. https://doi.org/10.1016/10.1016/j.seppur.2021.118889
Yu ZL, Qin B, Ma ZY, Gao YC, Guan QF, Yang HB, Yu SH (2020) Emerging bioinspired artificial woods. Adv Mater 23:2001086. https://doi.org/10.1016/10.1002/adma.202001086
Zhang XZ, Zhen DW, Liu FM, Chen R, Peng QR, Wang ZY (2023) An achieved strategy for magnetic biochar for removal of tetracyclines and fluoroquinolones: adsorption and mechanism studies. Bioresour Technol 369:128440. https://doi.org/10.1016/10.1016/j.biortech.2022.128440
Zhao HX, Lang YH (2018) Adsorption behaviors and mechanisms of florfenicol by magnetic functionalized biochar and reed biochar. J Taiwan Inst Chem Eng 88:152–160. https://doi.org/10.1016/10.1016/j.jtice.2018.03.049
Zhou CY, Li XC, Jiang HL, Ding Y, He GH, Guo J, Chu Z, Yu GH (2021a) Pulverizing Fe2O3 nanoparticles for developing Fe3C/N-Codoped carbon Nanoboxes with multiple polysulfide anchoring and converting activity in Li-S batteries. Adv Funct Mater 31:2011249. https://doi.org/10.1016/10.1002/adfm.202011249
Zhou Z, Cao JP, Wu Y, Zhuang QQ, Zhao XY, Wei YL, Bai HC (2021b) Waste sugar solution polymer-derived N-doped carbon spheres with an ultrahigh specific surface area for superior performance supercapacitors. Int J Hydrog Energy 46:22735–22746. https://doi.org/10.1016/10.1016/j.ijhydene.2021.04.126
Acknowledgements
The authors acknowledged the anonymous reviewers for comments to improve the quality of this work.
Funding
This work was supported by the National Natural Science Foundation of China (No: 21908242) and the Foundation of Science and Technology Project of Xuzhou City (KC22044).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Conceptualization, validation, review and editing were performed by Fu Chen. Material preparation, data collection and analysis were performed by Chenxi Zhu and Anhu Wang. Project administration, funding acquisition, review and editing were performed by Huagen Liang. The first draft of the manuscript was written by Huagen Liang and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors disclaim any financial conflicts of interest.
Additional information
Handling Editor: Baoshan Xing
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Liang, H., Zhu, C., Wang, A. et al. Facile preparation of NiFe2O4/biochar composite adsorbent for efficient adsorption removal of antibiotics in water. Carbon Res. 3, 2 (2024). https://doi.org/10.1007/s44246-023-00094-w
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s44246-023-00094-w