Abstract
Monitoring small amount of endocrine disrupting chemical, estradiol (E2) residue in environmental and biological samples is extremely important because of its possible connections to breast and prostate malignancies and gastrointestinal disorders. The newly synthesized graphene-coated silver nanoparticles (GN@Ag) decorated on graphitic carbon nitride (g-C3N4)-based hybrid nanomaterial (GN@Ag/g-C3N4) was used to modify glassy carbon electrode (GCE) for electroanalytical measurement of E2. The GN@Ag/g-C3N4 nanocomposite prepared through ultrasonic-assisted reflux methodology was characterized using various physicochemical methods. The scanning electron microscopy and transmission electron microscopy have shown that GN@Ag nanoparticles were decorated and randomly dispersed over g-C3N4 sheets. The exceptional electrochemical response towards the oxidation of E2 was observed through cyclic voltammetry due to the quick electron transfer ability and superior conductivity of GN@Ag/g-C3N4/GCE. The detection limit was found to be 0.002 μM with wide linear range of E2 concentration (0.005–8.0 μM) along with remarkable stability of the fabricated electrode for 21 days showing 91% retention in initial current. The kinetic parameters such as catalytic rate constant and diffusion coefficient for E2 were estimated to be 1.1 × 105 M−1 s−1 and 1.9 × 10−4 cm2 s−1, respectively, by employing chronoamperometry. The proposed sensor also demonstrated its practical applicability for E2 determination in environmental and biological samples with a recovery range of 95–104%. Furthermore, the developed sensing platform is much better compared to reported methods in terms of simplicity, accuracy, detection limit, linearity range, and usefulness in real sample for E2 sensing.
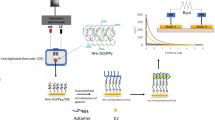
Graphical abstract









Similar content being viewed by others
Data availability
Most data generated or analyzed during this study are included in this published article and its supplementary information file.
References
Adeel M, Song X, Wang Y et al (2017) Environmental impact of estrogens on human, animal and plant life: a critical review. Environ Int 99:107–119. https://doi.org/10.1016/J.ENVINT.2016.12.010
Agrahari S, Kumar Gautam R, Kumar Singh A, Tiwari I (2022) Nanoscale materials-based hybrid frameworks modified electrochemical biosensors for early cancer diagnostics: an overview of current trends and challenges. Microchem J 172:106980. https://doi.org/10.1016/J.MICROC.2021.106980
Alam AU, Qin Y, Catalano M et al (2018) Tailoring MWCNTs and β-cyclodextrin for sensitive detection of acetaminophen and estrogen. ACS Appl Mater Interfaces 10:21411–21427. https://doi.org/10.1021/acsami.8b04639
Antoniazzi C, Alves De Lima C, Marangoni R et al (2018) Voltammetric determination of 17β-estradiol in human urine and buttermilk samples using a simple copper(II) oxide-modified carbon paste electrode. J Solid State Electrochem 22:1373–1383. https://doi.org/10.1007/s10008-017-3690-4
Arvand M, Hemmati S (2017) Analytical methodology for the electro-catalytic determination of estradiol and progesterone based on graphene quantum dots and poly(sulfosalicylic acid) co-modified electrode. Talanta 174:243–255. https://doi.org/10.1016/J.TALANTA.2017.05.083
Baghayeri M, Amiri A, Farhadi S (2016) Development of non-enzymatic glucose sensor based on efficient loading Ag nanoparticles on functionalized carbon nanotubes. Sensors Actuators B Chem 225:354–362. https://doi.org/10.1016/J.SNB.2015.11.003
Bhujel R, Rai S, Mustafa Z et al (2020) Synthesis and characterization of graphene sheet decorated with silver nanoparticles. AIP Conf Proc 2273:040002. https://doi.org/10.1063/5.0024234
Brocenschi RF, Rocha-Filho RC, Li L, Swain GM (2014) Comparative electrochemical response of estrone at glassy-carbon, nitrogen-containing tetrahedral amorphous carbon and boron-doped diamond thin-film electrodes. J Electroanal Chem 712:207–214. https://doi.org/10.1016/j.jelechem.2013.11.014
Cao Y, McDermott MT (2018) A surface plasmon resonance based inhibition immunoassay for measurement of steroid hormones. Anal Biochem 557:7–12. https://doi.org/10.1016/j.ab.2018.06.027
Cincotto FH, Martínez-García G, Yáñez-Sedeño P et al (2016) Electrochemical immunosensor for ethinylestradiol using diazonium salt grafting onto silver nanoparticles-silica–graphene oxide hybrids. Talanta 147:328–334. https://doi.org/10.1016/J.TALANTA.2015.09.061
Duan D, Si X, Ding Y et al (2019) A novel molecularly imprinted electrochemical sensor based on double sensitization by MOF/CNTs and Prussian blue for detection of 17β-estradiol. Bioelectrochemistry 129:211–217. https://doi.org/10.1016/J.BIOELECHEM.2019.04.014
Fekry AM (2017) A new simple electrochemical Moxifloxacin Hydrochloride sensor built on carbon paste modified with silver nanoparticles. Biosens Bioelectron 87:1065–1070. https://doi.org/10.1016/J.BIOS.2016.07.077
Fekry AM, Abdel-Gawad SA, Tammam RH, Zayed MA (2020) An electrochemical sensor for creatinine based on carbon nanotubes/folic acid/silver nanoparticles modified electrode. Measurement 163:107958. https://doi.org/10.1016/J.MEASUREMENT.2020.107958
Fröschl T, Hörmann U, Kubiak P et al (2012) High surface area crystalline titanium dioxide: potential and limits in electrochemical energy storage and catalysis. Chem Soc Rev 41:5313–5360. https://doi.org/10.1039/c2cs35013k
He Q, Yuan S, Chen C, Hu S (2003) Electrochemical properties of estradiol at glassy carbon electrode modified with nano-Al2O3 film. Mater Sci Eng C 23:621–625. https://doi.org/10.1016/S0928-4931(03)00053-5
He Y, Xie S, Yang X et al (2015) Electrochemical peptide biosensor based on in situ silver deposition for detection of prostate specific antigen. ACS Appl Mater Interfaces 7:13360–13366. https://doi.org/10.1021/acsami.5b01827
Hu S, Wu K, Yi H, Cui D (2002) Voltammetric behavior and determination of estrogens at Nafion-modified glassy carbon electrode in the presence of cetyltrimethylammonium bromide. Anal Chim Acta 464:209–216. https://doi.org/10.1016/S0003-2670(02)00496-8
Huang H, Shi S, Gao X et al (2016) A universal label-free fluorescent aptasensor based on Ru complex and quantum dots for adenosine, dopamine and 17β-estradiol detection. Biosens Bioelectron 79:198–204. https://doi.org/10.1016/J.BIOS.2015.12.024
Jaiswal N, Tiwari I (2021) Self-assembled benzoic acid functionalized graphene oxide sheets with zinc (II) ions: graphene oxide framework; novel material for environmental sensing application. Synth Met 276:116754. https://doi.org/10.1016/j.synthmet.2021.116754
Jaiswal N, Tiwari I, Foster CW, Banks CE (2017) Highly sensitive amperometric sensing of nitrite utilizing bulk-modified MnO2 decorated graphene oxide nanocomposite screen-printed electrodes. Electrochim Acta 227:255–266. https://doi.org/10.1016/j.electacta.2017.01.007
Janegitz BC, Dos Santos FA, Faria RC, Zucolotto V (2014) Electrochemical determination of estradiol using a thin film containing reduced graphene oxide and dihexadecylphosphate. Mater Sci Eng C 37:14–19. https://doi.org/10.1016/j.msec.2013.12.026
Kou BB, Zhang L, Xie H et al (2016) DNA enzyme-decorated DNA nanoladders as enhancer for peptide cleavage-based electrochemical biosensor. ACS Appl Mater Interfaces 8:22869–22874. https://doi.org/10.1021/acsami.6b07017
Kuhl H (2005) Pharmacology of estrogens and progestogens: influence of different routes of administration. Climacteric 8:3–63. https://doi.org/10.1080/13697130500148875
Kumar A, Kumar P, Joshi C, Boukherroub R (2016) Nickel decorated on phosphorous-doped carbon nitride as an efficient photocatalyst for reduction of nitrobenzenes nickel decorated on phosphorous-doped carbon nitride as an efficient photocatalyst for reduction of nitrobenzenes. Nanomaterials 6:59. https://doi.org/10.3390/nano6040059
Kumar D, Tiwari R, Patel RK et al (2021) One-pot synthesis of electroconducting graphene coated silver nanoparticles from silver acetylide. J Nanoparticle Res 23:1–12. https://doi.org/10.1007/s11051-021-05291-5
Li Y, Zhang H, Wu B, Guo Z (2017) Improving the oxidation resistance and stability of Ag nanoparticles by coating with multilayered reduced graphene oxide. Appl Surf Sci 425:194–200. https://doi.org/10.1016/J.APSUSC.2017.07.054
Lin X, Li Y (2006) A sensitive determination of estrogens with a Pt nano-clusters/multi-walled carbon nanotubes modified glassy carbon electrode. Biosens Bioelectron 22:253–259. https://doi.org/10.1016/j.bios.2006.01.005
Liu J, Zhang T, Wang Z et al (2011) Simple pyrolysis of urea into graphitic carbon nitride with recyclable adsorption and photocatalytic activity. J Mater Chem 21:14398–14401. https://doi.org/10.1039/c1jm12620b
Liu Z, Lau SP, Yan F (2015) Functionalized graphene and other two-dimensional materials for photovoltaic devices: device design and processing. Chem Soc Rev 44:5638–5679. https://doi.org/10.1039/c4cs00455h
Masikini M, Ghica ME, Baker PGL et al (2019) Electrochemical sensor based on multi-walled carbon nanotube/gold nanoparticle modified glassy carbon electrode for detection of estradiol in environmental samples. Electroanalysis 31:1925–1933. https://doi.org/10.1002/elan.201900190
Maślana K, Kaleńczuk RJ, Zielińska B, Mijowska E (2020) Synthesis and characterization of nitrogen-doped carbon nanotubes derived from g-C3N4. Materials (Basel) 13. https://doi.org/10.3390/ma13061349
Migowska N, Caban M, Stepnowski P, Kumirska J (2012) Simultaneous analysis of non-steroidal anti-inflammatory drugs and estrogenic hormones in water and wastewater samples using gas chromatography–mass spectrometry and gas chromatography with electron capture detection. Sci Total Environ 441:77–88. https://doi.org/10.1016/J.SCITOTENV.2012.09.043
Moraes FC, Rossi B, Donatoni MC et al (2015) Sensitive determination of 17β-estradiol in river water using a graphene based electrochemical sensor. Anal Chim Acta 881:37–43. https://doi.org/10.1016/J.ACA.2015.04.043
Moreira F, Santana ER, Spinelli A (2020) Ionic liquid-supported magnetite nanoparticles as electrode modifier materials for estrogens sensing. Sci Rep 10:1–11. https://doi.org/10.1038/s41598-020-58931-6
Ngundi MM, Sadik OA, Yamaguchi T, Suye S ichiro (2003) First comparative reaction mechanisms of β-estradiol and selected environmental hormones in a redox environment. Electrochem commun 5:61–67. https://doi.org/10.1016/S1388-2481(02)00538-6
Ong WJ, Tan LL, Chai SP et al (2015) Surface charge modification via protonation of graphitic carbon nitride (g-C3N4) for electrostatic self-assembly construction of 2D/2D reduced graphene oxide (rGO)/g-C3N4 nanostructures toward enhanced photocatalytic reduction of carbon dioxide to methane. Nano Energy 13:757–770. https://doi.org/10.1016/J.NANOEN.2015.03.014
Pandiyarajan C, Rameshkumar P, Murugesan S, Selvaraj M (2021) Silver nanoparticles-supported graphitic-like carbon nitride for the electrochemical sensing of nitrobenzene and its derivatives. J Mater Sci Mater Electron 32:19912–19924. https://doi.org/10.1007/s10854-021-06515-z
Raj M, Goyal RN (2019) Silver nanoparticles and electrochemically reduced graphene oxide nanocomposite based biosensor for determining the effect of caffeine on estradiol release in women of child-bearing age. Sensors Actuators B Chem 284:759–767. https://doi.org/10.1016/J.SNB.2019.01.018
Rajavel K, Lalitha M, Radhakrishnan JK et al (2015) Multiwalled carbon nanotube oxygen sensor: enhanced oxygen sensitivity at room temperature and mechanism of sensing. ACS Appl Mater Interfaces 7:23857–23865. https://doi.org/10.1021/acsami.5b04869
Rameshkumar P, Ramaraj R (2014) Electroanalysis of nitrobenzene derivatives and nitrite ions using silver nanoparticles deposited silica spheres modified electrode. J Electroanal Chem 731:72–77. https://doi.org/10.1016/J.JELECHEM.2014.08.010
Ray JA, Kushnir MM, Bunker A et al (2012) Direct measurement of free estradiol in human serum by equilibrium dialysis–liquid chromatography–tandem mass spectrometry and reference intervals of free estradiol in women. Clin Chim Acta 413:1008–1014. https://doi.org/10.1016/J.CCA.2012.02.028
Salehnia F, Hosseini M, Ganjali MR (2017) A fluorometric aptamer based assay for cytochrome C using fluorescent graphitic carbon nitride nanosheets. Microchim Acta 184:2157–2163. https://doi.org/10.1007/s00604-017-2130-6
Saravanakumar K, Karthik R, Chen S et al (2017) Construction of novel Pd/CeO 2/g-C 3 N 4 nanocomposites as efficient visible-light photocatalysts for hexavalent chromium detoxification Journal of Colloid and Interface Science Construction of novel Pd / CeO 2/g-C 3 N 4 nanocomposites as efficient. J Colloid Interface Sci 504:514–526. https://doi.org/10.1016/j.jcis.2017.06.003
Shanmugam R, Alagumalai K, Chen SM, Ganesan T (2021) Electrochemical evaluation of organic pollutant estradiol in industrial effluents. J Environ Chem Eng 9:105723. https://doi.org/10.1016/j.jece.2021.105723
Shi Y, Peng DD, Shi CH et al (2011) Selective determination of trace 17β-estradiol in dairy and meat samples by molecularly imprinted solid-phase extraction and HPLC. Food Chem 126:1916–1925. https://doi.org/10.1016/j.foodchem.2010.12.020
Singh AK, Jaiswal N, Gautam RK, Tiwari I (2021) Development of g-C3N4/Cu-DTO MOF nanocomposite based electrochemical sensor towards sensitive determination of an endocrine disruptor BPSIP. J Electroanal Chem 887:115170. https://doi.org/10.1016/j.jelechem.2021.115170
Song J, Yang J, Hu X (2008) Electrochemical determination of estradiol using a poly(L-serine) film-modified electrode. J Appl Electrochem 38:833–836. https://doi.org/10.1007/s10800-008-9520-8
Spina RL, Ferrero VEV, Aiello V et al (2017) Label-free biosensor detection of endocrine disrupting compounds using engineered estrogen receptors. Biosensors 8:1–15. https://doi.org/10.3390/bios8010001
Wang C, Li J, Luo X et al (2016) Graphitic carbon nitride nanosheets modified multi-walled carbon nanotubes as 3D high efficient sensor for simultaneous determination of dopamine, uric acid and tryptophan. J Electroanal Chem 780:147–152. https://doi.org/10.1016/J.JELECHEM.2016.09.004
Wang W, Ge L, Sun X et al (2015) Graphene-assisted label-free homogeneous electrochemical biosensing strategy based on aptamer-switched bidirectional DNA polymerization. ACS Appl Mater Interfaces 7:28566–28575. https://doi.org/10.1021/acsami.5b09932
Wang Z, Wang P, Tu X et al (2014) A novel electrochemical sensor for estradiol based on nanoporous polymeric film bearing poly{1-butyl-3-[3-(N-pyrrole)propyl]imidazole dodecyl sulfonate} moiety. Sensors Actuators B Chem 193:190–197. https://doi.org/10.1016/J.SNB.2013.11.053
Zhang W, Sherrell P, Minett AI et al (2010) Carbon nanotube architectures as catalyst supports for proton exchange membrane fuel cells. Energy Environ Sci 3:1286–1293. https://doi.org/10.1039/c0ee00139b
Zhao Q, Wu W, Wei X et al (2017a) Sensors and actuators B : chemical graphitic carbon nitride as electrode sensing material for tetrabromobisphenol-A determination. Sensors Actuators B Chem 248:673–681. https://doi.org/10.1016/j.snb.2017.04.002
Zhao Q, Zhou H, Wu W et al (2017b) Sensitive electrochemical detection of tetrabromobisphenol A based on poly(diallyldimethylammonium chloride) modified graphitic carbon nitride-ionic liquid doped carbon paste electrode. Electrochim Acta 254:214–222. https://doi.org/10.1016/J.ELECTACTA.2017.09.114
Zhou X, Wang A, Yu C et al (2015) Facile synthesis of molecularly imprinted graphene quantum dots for the determination of dopamine with affinity-adjustable. ACS Appl Mater Interfaces 7:11741–11747. https://doi.org/10.1021/am5078478
Zhu J, Xiao P, Li H, Carabineiro SAC (2014) Graphitic carbon nitride: synthesis, properties, and applications in catalysis. ACS Appl Mater Interfaces 6:16449–16465. https://doi.org/10.1021/am502925j
Zhu Y, Liu X, Jia J (2015) Electrochemical detection of natural estrogens using a graphene/ordered mesoporous carbon modified carbon paste electrode. Anal Methods 7:8626–8631. https://doi.org/10.1039/c5ay01833a
Acknowledgements
A. K. S. (Chem./2018-19/RET/Sept.18-term/1/4809), S. A. (Chem./2019-2020/RET-2/Sept.19-term/1/975), and R. K. G. (D.S. Kothari PDF No. F. 4-2/2006(BSR)/CH/17-18/271) are thankful to UGC, New Delhi for providing their research fellowships. Prof. N. V. Chalapathi Rao, Department of Geology, BHU, is thanked for providing SEM and EDX facilities. Department of Physics, BHU, is thanked for providing TEM facilities.
Funding
This study is financially supported by The Scheme for Promotion of Academic and Research Collaboration (SPARC-6019), MHRD, India and IOE incentive grant for faculty (Scheme Number-6031), BHU.
Author information
Authors and Affiliations
Contributions
AKS: methodology, conceptualization, visualization, writing—original draft; SA: writing—review and editing, data curation; RKG: investigation, formal analysis; IT: supervision, validation
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Ester Heath
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• Coating of graphene (GN) on silver nano particles (Ag NPs) prevents their agglomerations.
• Immobilization of graphene-coated Ag NPs on graphitic carbon nitride (g-C3N4) enhances the electrical conductivity and electrocatalytic activity.
• Fabrication of GN@Ag/g-C3N4 nanocomposite modified glassy carbon electrode (GCE) as a novel sensing platform for electrochemical determination of estradiol.
• GN@Ag/g-C3N4/GCE exhibits a detection limit at nanomolar level with wide linear response.
• GN@Ag/g-C3N4/GCE shows excellent repeatability, reproducibility, and stability.
Supplementary information
ESM 1
(DOCX 969 kb)
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Singh, A.K., Agrahari, S., Gautam, R.K. et al. Fabrication of an innovative electrochemical sensor based on graphene-coated silver nanoparticles decorated over graphitic carbon nitride for efficient determination of estradiol. Environ Sci Pollut Res (2022). https://doi.org/10.1007/s11356-022-23410-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11356-022-23410-0