Abstract
Hydrogen energy, as clean and efficient energy, is considered significant support for the construction of a sustainable society in the face of global climate change and the looming energy revolution. Hydrogen is one of the most important chemical substances on earth and can be obtained through various techniques using renewable and nonrenewable energy sources. However, the necessity for a gradual transition to renewable energy sources significantly hampers efforts to identify and implement green hydrogen production paths. Therefore, this paper’s objective is to provide a technological review of the systems of hydrogen production from solar and wind energy utilizing several types of water electrolyzers. The current paper starts with a short brief about the different production techniques. A detailed comparison between water electrolyzer types and a complete illustration of hydrogen production techniques using solar and wind are presented with examples, after which an economic assessment of green hydrogen production by comparing the costs of the discussed renewable sources with other production methods. Finally, the challenges that face the mentioned production methods are illuminated in the current review.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Due to increased world populations, the extensive exploration and use of fossil fuels have led to several environmental issues harming human health and life (Khan et al. 2022). Therefore, current primary concerns have included ways to provide a cost-effective, dependable, and environmentally friendly primary energy source with as low carbon emissions as possible. Furthermore, this energy source should be sustainable and accessible in every region (Chien et al. 2021) (Eldesoukey and Hassan 2019). Hence, there is an urgent need to find and use an alternative clean energy source that is renewable and safe enough to replace nonrenewable sources. However, abundant difficulties are inherent in renewable energy power plants (Zhang et al. 2006; Abdelshafy et al. 2018). For example, these plants are installed in arid regions and need a storage system due to the intermittent nature of renewable sources (Singla et al. 2021).
Based on these issues, hydrogen, which is considered an alternative energy carrier, is proposed to play a significant role in future energy because it can be stored and transported and has a high calorific combustion value, making it suitable to replace fossil fuels (Saxena et al. 2008). Its eco-friendly production process also accounts for one of its key features on the road to a better environment and the success of sustainable development (Joshi et al. 2010). Moreover, hydrogen can be directly applied to fuel cells to produce electricity without any toxic emissions but with an energy yield of about 122 KJ/g, which is 2.75 times greater than hydrocarbon fuels (Fan et al. 2021). Table 1 shows the thermophysical properties of hydrogen.
Therefore, this paper provides a general overview of the hydrogen production techniques according to feedstock type and energy source, focusing on hydrogen production systems from water electrolysis using solar and wind energy. Furthermore, a detailed comparison between different electrolyzer types was conducted, focusing on their advantages and disadvantages. In the final section, an economic assessment of the understudied production system is then illustrated to show the hydrogen production costs and challenges that face each technique. From the details of this paper, we propose to help researchers develop a good understanding of clean hydrogen production techniques through water electrolysis using wind and solar since these sources have been on an upward curve since 2000, as illustrated in Fig. 1. This figure demonstrates the number of published articles per year, which are in ascending trend because of the increased interest in alternative energy sources. It is noted that solar energy is superior to wind power in terms of hydrogen production.
Number of published articles on hydrogen production using solar and wind energy (Elsevier 2022)
Alternatively, although solar energy is superior to wind power in hydrogen production, electrolysis generally has significant downsides, such as when using platinum-based electrocatalytic metals or due to high energy demands and observed corrosion at the cathode. Hence, several patent innovations have been primarily proposed concerning the search for electrode material enhancements (Martinez-Burgos et al. 2021). To this end, the green hydrogen production process has accounted for increasing patents since 2005, with the number of patents in 2005 being 55 but increasing to 375 inventions in 2020 (an increase of 588%). Notably, Japan and the United States have a considerable lead in the number of innovations (IRENA 2022).
Hydrogen production technologies
The hydrogen industry is divided into four major parts: production, storage, transportation, and use (Fig. 2). As shown in Fig. 2, while natural gas is the primary source of hydrogen production, ammonia manufacturing is the most hydrogen-consuming industry. Furthermore, hydrogen transportation from the production site to the consumption site is a vital process in the hydrogen production economies because it can increase production costs, influencing its affordability. Hydrogen storage is considered an urgent and challenging stage because it helps develop safe, reliable, efficient, and adequate storage mechanisms (Zhang et al. 2016). Therefore, hydrogen production processes based on feedstocks have also been proposed. Table 2 briefly explains the hydrogen production processes, focusing on water electrolysis.
Water electrolyzer
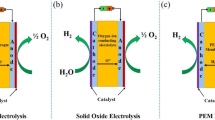
Two essential components of a green hydrogen system are its renewable energy source and the use of a water electrolyzer. During water electrolysis, water decomposes into hydrogen and oxygen under electricity using an electrolyzer. Therefore, due to its intermittency, this electrolyzer has been proposed as the most feasible and commercial method for hydrogen production and energy storage when coupled with renewable energy. Based on these facts, the most common electrolyzers are the proton exchange membrane electrolyzer (PEM), alkaline water electrolyzer (AWE), alkaline anion exchange membrane (AEM), and solid oxide electrolyzer (SOE) (Chi and Yu 2018; Lim and Kim 2022). Table 3 demonstrates the main points distinguishing each electrolyzer to show the difference between each type. The main benefits of renewable energy/water electrolyzers are as follows: (1) the possible reduction or elimination of transportation and storage costs because they can be used as stand-alone systems for end-user sites, (2) their compactness and the possibility of high hydrogen production against photo-electrochemical, (3) lack of electricity infrastructural needs in arid regions, and (4) their commercially available nature (Nasser et al. 2022a).
Electrolyzer stacks comprise many connected cells, categorized into monopolar and bipolar types. While the bipolar design connects the cells in series, the monopolar cells are connected electrically and geometrically in parallel. Consequently, electric wiring is less in the bipolar one due to its compactness, enhancing its efficiency. However, this type’s main drawback is its high cost due to its complex design compared with monopolar stacks (Zhang et al. 2016).
Proton exchange membrane electrolyzer (PEM)
A PEM electrolyzer was firstly introduced in the 1960s by General Electric (Buttler and Spliethoff 2018). The fundamental components of PEM are its anode, cathode, and electrolyte (Table 3), while the most common materials for anode and cathode are platinum, iridium, ruthenium, and platinum on carbon. The Chemours Company FC, LLC, with trademark Nafion and FUMATECH BWT GmbH with trademark Fumapem are the typical suppliers for the PEM membrane. Alternatively, electrolyte materials are responsible for the high conductivity of protons, low gas crossover, compact design of an electrolyzer, and high operation pressure (15–30 bar at 50–90°C) (Carmo et al. 2013).
The significant PEM advantages are that it can perfectly deal with load fluctuation due to its rapid response, with its produced hydrogen purity up to 99.999% (Buttler and Spliethoff 2018). In contrast, the main disadvantage, until now, is its high cost due to the noble material used inside the electrolyzer (Bhandari et al. 2014). Table 3 shows PEM’s operation principle and main parameters.
Alkaline water electrolyzer (AWE)
AWE is the most mature technology among the other types. It is reliable and safe and can be maintained in a large-scale unit (Yan and Hino 2018). This electrolyzer is composed of two electrodes submerged in a liquid electrolyte water solution, usually 20–40% sodium hydroxide (NaOH) or potassium hydroxide (KOH) (Zhang et al. 2016). A diaphragm separates these electrodes in the solution, allowing water molecules and hydroxide ions to pass through. The diaphragm also separates H2 and O2 for safety and purity aspects (Carmo et al. 2013; El-Emam and Özcan 2019) (Table 3). Consequently, the purity of the produced hydrogen is 99.5 to 99.9% and can be increased up to 99.999% by catalytic gas purification processes (Buttler and Spliethoff 2018).
Notably, AWE performance is influenced by the diaphragm, anode, and cathode material type and thickness. As an example, Fig. 3 demonstrates the effect of cathode material on hydrogen production (Mert et al. 2019). Investigations revealed that while the Cu/NiMo cathode has the highest hydrogen production, the Cu cathode has the lowest. Moreover, although an electrolyte’s temperature does not affect hydrogen production and lowers the required power (Rahim et al. 2015), an electrolyte solution’s concentration affects the output. Therefore, the main difference between PEM and AWE is the electrolyte type since PEMs use a solid polymer membrane electrolyte, but AWEs use a corrosive liquid electrolyte.
Hydrogen production from AWE at different cathode materials (Mert et al. 2019)
Alkaline anion exchange membrane (AEM)
AEM has recently been designed as an alternative to traditional water electrolyzers. Interestingly, AWE and PEM are combined in AEM to address some of the drawbacks of the first and second electrolyzer types. Hence, it combines a low-concentration alkaline solution as opposed to a 20–40% KOH or NaOH aqueous solution with a solid electrolyte (polymeric) membrane (e.g., Mg-Al LDH) (Cho et al. 2018; Li and Baek 2021). Furthermore, the anode in an AEM is manufactured from Ni-based (e.g., Ni foams) or titanium materials, and the cathode comprises Ni, Ni-Fe, and NiFe2O4 (Faid et al. 2018; Chi and Yu 2018; Li and Baek 2021). Table 3 shows the reaction inside AEM and its working principle.
Solid oxide electrolyzer (SOE)
Although SOE operates at a high temperature, the electricity required to drive its electrolysis process at such a high temperature is significantly reduced compared to low-temperature electrolysis. Therefore, the system’s efficiency is improved because it uses inexpensive thermal energy or waste heat. Furthermore, while the cathode material is made from a 50/50 wt% mixture of lanthanum strontium manganite and yttrium-stabilized zirconia, the anode and electrolyte materials are cermets and ceramic, respectively (El-Emam and Özcan 2019). However, SOE must undergo further research and development to provide better catalyst and electrode materials (El-Emam and Özcan 2019).
Its hydrogen production process is described as follows: First, steam at the cathode side is reduced to hydrogen according to the cathode reaction, and then, the oxide anions generated on the cathode side are the path through which solid electrolytes form oxygen on the anode side. Table 3 summarizes SOE’s characteristics, specifications, advantages, and disadvantages.
Challenges of water electrolysis
The primary goal of commercializing hydrogen generation using electrolysis is to reduce investment and operational expenses (Younas et al. 2022). While it is possible to build renewable water electrolysis systems using currently available technologies, the system’s costs are unlikely to decrease soon without a dramatic breakthrough in solar and wind technology. Other issues, such as the intermittent nature of energy sources, water consumption rates, and their efficiencies, also need to be addressed. Therefore, this electrolysis method is considered less attractive, considering its hydrogen production cost.
Green electricity production systems
Solar and wind energy produces sufficient electricity to drive electrolyzers for hydrogen storage or direct use during production. Typical examples of solar energy are photovoltaic (PV) and concentrated solar power (CSP) systems (Soliman et al. 2019). However, wind turbines used to convert wind to power are an example of wind energy. Although PV panels and wind turbines are directly coupled with electrolyzers, CSP is first associated with a power cycle for electricity production before connecting them to electrolyzers. Therefore, the need for an AC/DC or DC/DC converter is mandatory in electrolyzer load adjustments.
Green production offers an ideal solution to provide remote areas with power due to the high cost of power transmission (Singla et al. 2021). Therefore, excess energy from renewable sources has been used to operate electrolyzers for hydrogen production. Hydrogen can also be used in fuel cells to produce electricity during the night or intermittency. Fig. 4 presents the basic concept of a solar/wind hydrogen production system.
In a PV/hydrogen production (PV/H2) system, PV panels are linked to an electrolyzer through a power-conditioning unit containing a maximum power point tracking (MPPT) system and a DC/DC converter. This unit is applied to maximize the output from panels and adjust the electrolyzer input power (Haider et al. 2021; Nasser et al. 2022b). However, in the case of excess electricity from PV systems, a battery system is adopted as energy storage. Therefore, the main benefits of the PV/H2 system over other systems are the use of a DC electricity output and the absence of moving parts, which leads to minor maintenance. Contrastively, in the CSP/hydrogen production (CSP/H2) system, solar radiation heat is divided into two portions: the first is used in power cycles (e.g., organic Rankine cycle (ORC)) to generate electricity that drives the electrolyzer, and the second converts water to steam by employing SOE, as mentioned in Fig. 4 (Chadegani et al. 2018). Based on this principle, the system may have thermal storage to ensure continuous production at night. A previous study compared PV/H2 and CSP/H2 under the same conditions to investigate their system performances (Joshi et al. 2011). The results revealed that the CSP/H2 system performed better than PV/H2.
Comparatively, the wind/hydrogen production (wind/H2) system is more like a PV/H2 system but needs an AC/DC converter to drive the electrolyzer. Although wind energy is available throughout the day in contrast to solar energy, this system has a significant weakness of wind’s unpredictable nature. Fig. 4 illustrates the essential components of this system. Interestingly, a combination of these energy sources can be applied to enhance a system’s efficiency and provide a multi-generation cycle. Therefore, this possibility is discussed in detail in the current study.
The PV/H2 system
The PV/H2 system is a promising technique for green hydrogen production due to its economically competitive, commercially viable, and sustainable structure (Bhattacharyya et al. 2017). Therefore, this system has been investigated under different climatic conditions with and without a solar tracking system (Bilgen 2001). Notably, a study indicated that although the system with solar tracking had higher performance, its capital cost was raised. Moreover, using concentrated PV increased the system efficiency from 12 to 16% more than the non-concentrated one (Bicer and Dincer 2017). Hence, the mathematical study had the same results as the experiment (Ismail et al. 2019).
Furthermore, another study investigated the system’s performance with and without MPPT (Ganeshan et al. 2016). Their investigations revealed that although the system with direct coupling had an efficiency close to the system with MPPT, the capital cost of the system with MPPT was higher. Another study also mentioned that increasing MPPT efficiency increased hydrogen production (Rahim et al. 2015). Therefore, the PEM electrolyzer is suitable for PV/H2 due to its ability to deal with load fluctuation and its lowest cold startup property, among other types (Paul and Andrews 2008), as illustrated in Table 3.
Since early PV/H2 systems have low performances (2–6%) and high production costs (40 $/kg) (Gibson and Kelly 2008), PV panels and electrolyzer enhancements are employed to reduce costs with increasing production, which minimizes hydrogen production costs and enhances system performances. Based on this principle, a system’s efficiency was increased up to 12.4% instead of 6% by directly connecting PV with an electrolyzer (Gibson and Kelly 2010). Another study observed that hydrogen’s levelized cost (LCOH) ranged from 1.8 to 3.4 $/kg instead of 40 $/kg, which was mentioned before (Şevik 2022). This high efficiency was obtained when the output voltage from the PV panels was equal to or slightly higher than the required voltage for the electrolyzer. In yet another study, a PV/H2 with and without a battery system was compared to investigate their constant daily electric load consumption (Richards and Conibeer 2007). Investigations revealed that this system was more suitable as a stand-alone system in arid areas and areas with high elevations (Valdés et al. 2012). It was also reported that the tilted PV panels possessed higher efficiency during hydrogen production in a stand-alone system than horizontal panels (Tebibel 2017). However, the PV/H2 system without a battery requires fewer PV panels and involves low LCOH. Therefore, coupling PV panels with a water electrolyzer to produce the necessary hydrogen for fuel cells and provide electricity during the night or winter is a promising technique for the future (Lagorse et al. 2008).
Alternatively, purchasing electricity to operate the electrolyzer during solar radiation off-periods is economically viable due to the increase in working hours (Ferrari et al. 2016). The results demonstrated that since the LCOH values for grid/H2, grid+PV/H2, and PV/H2 were 5.5, 6.1, and 12.1 $/kg, respectively (Shaner et al. 2016; Grimm et al. 2020; Matute et al. 2022), the system’s cost could be recovered in 12 years. They also discovered that the enormous capital cost was mainly due to land costs and the amount used for PV panel construction. Therefore, remote areas with abundant solar radiation are considered more suitable locations for this type of plant. Since the optimal integration between PV panels and water electrolyzers is mandatory to provide high hydrogen production by increasing a system’s efficiency, monofacial and bifacial PV panels are used in PV/H2 systems to show their impact on efficiency under similar operating conditions (Privitera et al. 2020). Investigations revealed that the efficiency was up to 13.5% for bifacial PV panels rather than 11.55% for monofacial PV panels. Based on the bifacial PV, they also observed that hydrogen production increased from 3.7 to 4.2 g/h per square meter.
Remarkably, it was recently observed that an ultrahigh concentration PV/H2 system could improve a system’s efficiency to approximately 18–21% rather than 9.4% for conventional PV, with its hydrogen production ranging from 0.8 to 1.0 L/min per square meter for concentrated PV/H2 (Muhammad-Bashir et al. 2020). Although the battery increases the system capital cost, it reduces the electrolyzer’s size and enables hydrogen production at night, involving an LCOH around 6–7 €/kg (Gutiérrez-Martín et al. 2020; Zhang and Wei 2020; Puranen et al. 2021). Likewise, DCX converters use a high-efficiency DC voltage in PV/H2 to increase the system’s efficiency (Concha et al. 2021).
Since the performance of conventional PV panels increases by reducing their temperature, photovoltaic thermal (PVT) can introduce high-electricity output plus heat energy for several purposes (Li et al. 2022a) (Soliman and Hassan 2019). As a result, this electricity increases the hydrogen production from an electrolyzer, thereby reducing production costs. Furthermore, while air, water, and nanofluids as cooling fluids can reduce the PVT temperature, output fluid from PVT could be used for heating (Gado et al. 2022). Consequently, hydrogen production from coupling PV, PVT/air, and PVT/water with water electrolyzer reaches up to 8.19, 13.9, and 17.12 mL/min, respectively (Senthilraja et al. 2020), whereas PVT/nanofluids increase the power output by 47% compared with PVT/water (Sangeetha et al. 2021).
Multi-generation PV/H2 system
Electricity from PV panels can also produce hydrogen from electrolyzers in addition to other applications (e.g., space heating or cooling) (Erzen et al. 2020; Tukenmez et al. 2021; Şevik 2022). Therefore, these systems are currently attracting more attention due to their high performance compared to traditional PV/H2 systems. Table 4 summarizes the multi-generation techniques and their main specifications. Consequently, while the PV and PVT panels provide heat and electricity demand for buildings (Elghamry et al. 2019), their excess electricity drives the electrolyzer for hydrogen. Hydrogen notably drives fuel cells to provide heat, electricity, and sometimes drinkable water. Hence, in practice, using the Hoffmann voltammeter electrolyzer enables researchers to perform pretreatment for wastewater to reduce pollutants. Besides hydrogen production, the system can be used for ammonia production (Siddiqui and Dincer 2020). The results revealed that the system exergy efficiency reaches up to 55.5% and the produced ammonia is up to 1949.8 kmol, while hydrogen production is up to 5849.3 kmol.
The multi-generation system can use other energy sources besides solar (Hassan et al. 2022). For example, integrating PV panels with a mini-hydro plant for electricity production to drive an electrolyzer increases hydrogen production from 50,554 to 62,568 Nm3/year (Pereira et al. 2017). As a result, while electricity from the PV was 22.5% of the total produced electricity, the remaining part was from a mini-hydro plant. Furthermore, integrating a flat plate collector with the ocean thermal energy conversion (OTEC) cycle to produce electricity for the water electrolyzer has also been introduced (Yilmaz et al. 2018). Investigations proved that while the exergonic efficiency ranged from 22 to 36.49%, hydrogen production was about 1.2 Kg/h.
PV/H2 case studies
Several case studies have been conducted to estimate and analyze hydrogen production amounts, including the cost of producing 1 kg of hydrogen using PV panels under different climatic conditions (e.g., Algeria and Morocco). For example, a numerical study was conducted in the United States to show the ability of the PV/H2 system to supply the first two fuel cell buses with hydrogen (Vidueira et al. 2003). Their investigations revealed that the system could provide the buses with enough hydrogen to operate all day without emission. In other studies, while the potential for hydrogen production in southern Algerian regions proved better than in the northern areas by 45% under the same conditions (Mokhtara et al. 2020; Khelfaoui et al. 2020), other regions of Algeria recorded a higher hydrogen production than North African countries (Saadi et al. 2016). For instance, under the Moroccan climate, LCOH ranged from 4.64 to 5.79 $/kg when the electricity cost from PV panels was 0.077–0.099 $/kWh (Touili et al. 2018). Hence, a study compared fixed PV, PV with a tracking system, and a Stirling dish for H2 production in some regions (Touili et al. 2020). Their results confirmed that while the LCOH during fixed PV was the lowest, reaching up to 5.8 $/kg, the Stirling dish/H2 had the highest efficiency. However, this cost was lower than 5.96 $/kg in Southern Spain, 6.51 $/kg for South Africa, and 6.6 $/kg for the United States (Koleva et al. 2021). Another study used 400-watt PV panels in Cotonou, Benin. The hydrogen production was 115 L/day for 1.09 €/m3 based on their climatic conditions (Fopah-Lele et al. 2021).
CSP/H2 system
As previously stated, the CSP/H2 system generates electricity and heat to operate the electrolyzer and other methods. In such a system, solar radiation is collected and concentrated using a solar collector (e.g., flat plate collector (FPC) and parabolic dish collector (PDC)). While the generated heat is employed to drive the power cycle for electricity production, another portion is used to produce steam, like in the case of SOE, or to power absorption cooling cycles, as presented in Fig. 5. The power cycles used in this system are organic Rankine cycle (ORC) and Brayton cycle. Mainly, ORC attracts more attention because it works with low-grade heat. Table 5 summarizes the main specifications for a CSP/H2 system.
The schematic diagram of the CSP/H2 multi-generation system (Chadegani et al. 2018)
A CSP/H2 system can also be used for hydrogen production, electricity production, heating, cooling, and freshwater supply (Fig. 5). These multi-generation techniques enhance the total system’s efficiency (Chadegani et al. 2018). To this end, a study reported that the energy and exergy efficiency of a CSP/H2 system could vary from 33.52 to 71.6% and 20.7 to 36%, respectively (Delpisheh et al. 2021; Gill et al. 2021). It was also reported that a CSP/H2 multi-generation system’s overall efficiency increases when the solar radiation improves, lowering the electrolyzer’s working temperature and growing its current density (Chen et al. 2020). Hence, these systems could reduce CO2 emissions.
Notably, CSP/H2-based multi-generation systems also employ other energy sources (e.g., geothermal energy) to enhance the system’s efficiency and better use of available energy sources in regions (Sen et al. 2021; Temiz and Dincer 2021). To this end, a study reported that while LCOH reached up to 2.84 $/kg using a multi-generation system, the levelized cost of electricity (LCOE) reached 0.03 $/kWh. In yet another study, a Stirling engine was installed at the focus point of a solar concentrator to produce electricity directly, which regulated the water electrolyzer (Marefati et al. 2019; Zayed et al. 2021). Based on these facts, the amount of hydrogen produced using the PV and CSP/Stirling systems was compared (Lahoussine Ouali et al. 2020). Investigations revealed that the hydrogen produced was 302.2 kg for CSP/Stirling systems and 267.8 kg for PV/H2.
The wind/H2 system
Due to wind’s unpredictable and intermittent nature, wind turbines’ electricity fluctuates throughout their operational period. Therefore, a time exists in the operation period when the produced electricity is higher or lower than the required electric demand. During this period, excess electricity must be stored. To this end, the wind/H2 system has been proposed as a solution for long-term energy storage of electricity in the form of hydrogen gas because this gas converts to electricity again during low production periods, as demonstrated in Fig. 6. Subsequently, the electrolyzer can be operated based on the accumulated electricity from the wind turbine, or using the whole electricity from hydrogen production, the produced hydrogen can also be sold instead of converted to electricity (Carton and Olabi 2010; Xiao et al. 2020). Therefore, coupling a wind turbine with a water electrolyzer and a fuel cell provides a sustainable and clean energy solution (Dabar et al. 2022). Furthermore, combining wind energy and water electrolyzer also increases the performance of wind turbines and the produced hydrogen used as a backup unit (Liu et al. 2021).
A wind/H2 system is constructed by coupling a wind turbine generator with an AC/DC converter before attaching them to a water electrolyzer (Nadaleti et al. 2019). In this system, produced hydrogen is employed for several applications according to the following scenarios: The first is the wind/H2 grid–independent scenario, where the water electrolyzer is directly coupled with wind energy through a power-conditioning system. This scenario is more suitable in remote areas, where most wind farms are installed (Luo et al. 2022). The second scenario is the wind/H2 grid–assisted system, where the electricity from the grid near the wind farm is used to help the wind turbine produce hydrogen due to wind intermittency. While the third scenario involves wind energy that drives the electrolyzer during excess production and provides electricity to the grid, the fourth is similar to the previous one and contains a storage system and fuel cell for electricity production (Zhou and Francois 2009; Geovanni et al. 2010). Finally, the last one has a storage system and serves two purposes: (I) to power the fuel cell and produce electricity and (II) to transport part of the stored hydrogen for other purposes (Sherif et al. 2005). Any excess hydrogen produced can then be used in fueling stations and methanation processes. Fig. 6 shows the essential components of all scenarios.
Based on the principles above, a study coupled a horizontal axis wind turbine with AWE and a fuel cell to produce a constant electric supply in the Aegean islands (Iqbal 2003; Ntziachristos et al. 2005). Their results showed that the system’s overall efficiency reached 60% due to this configuration. For the first time, another study also showed that the wind/H2 system could provide ten households with electricity for 2–3 days (Ulleberg et al. 2010). However, the electricity cost of this system is incomparable to that of conventional ones. Nevertheless, cost implications are proposed to be reduced in the future due to the taxes on CO2 emissions and an increase in fossil fuel costs. A primary concern about the wind farm still persists: the power fluctuation changes based on wind speed. To this end, a novel switching strategy with a chopper circuit for each electrolyzer has been used to regulate the input electricity to the electrolyzer (Muyeen et al. 2011). The results showed that each electrolyzer worked full load, increasing its lifespan and efficiency. Another study that used a vertical axis wind turbine in the wind/H2 system has also reported acceptable performance (Demirdelen et al. 2020).
Notably, the wind/H2 system has been investigated numerically using four electrolyzer models (Sarrias-Mena et al. 2015). The investigations revealed that all models performed similarly under different wind speed conditions. Subsequently, the system’s performance was enhanced by combining more than one control method (Fang and Liang 2019; Qiu et al. 2019; Saenz-Aguirre et al. 2020). Other studies have also investigated the performance of a new wind/H2 system strategy (Grüger et al. 2019). The results showed that the production cost was reduced by 9%, from 13.28 to 11.52 €/kg. Similarly, the wind/H2 system was investigated under different capacity factors in Kuwait (Sedaghat et al. 2020). The results illustrated the system could produce 0.01 Kg/h when consuming 628.4 W from a 2-kW wind turbine (Shen et al. 2021).
Remarkably, studies have also proven that hydrogen production costs from wind/H2 systems vary from one place to another according to the amount and price of electricity produced from wind turbines and electrolyzer costs. For example, in Afghanistan, while the LCOH ranged from 2.118 to 2.261 $/kg, the LCOE was 0.063–0.079 $/kWh (Rezaei et al. 2020). However, in Yazd City, Iran, the LCOE and LCOH implications were 0.068–0.115 $/kWh and 2.1008–3.5602 $/kg, respectively (Almutairi et al. 2021). In contrast, while the LCOE and LCOH implications reduced to 0.0325–0.0755 $/kWh and 1.375–1.59 $/kg in Lutak City, Iran, due to the high wind speed conditions in this region (Rezaei et al. 2021), the LCOH was 3.1 and 4.02 $/kg in Turkey and Pakistan, respectively (Genç et al. 2012; Iqbal et al. 2019). Moreover, while the LCOH in South Africa was between 6.34 and 8.97 $/kg (Ayodele et al. 2021), it was 7.3 $/kg in Germany (Herwartz et al. 2021).
Hydrogen can be physically stored as a compressed gas in a storage tank under high pressure for transportation. However, the main concern with hydrogen storage is the leakage of compressed gas (Li et al. 2022b), as experiencing a high-pressure hydrogen leak can result in an explosion, leading to significant injuries and property damage. Accordingly, a previous study (Nasser et al. 2022a) established that the storage system raises hydrogen production costs due to the increased capital cost of system components. The results revealed that the production cost increased by 50% when a storage system was used. Based on this limitation, installing offshore wind turbines during hydrogen production from the water electrolyzer has been investigated under different scenarios (Franco et al. 2021). Investigations revealed that the LCOH implication was 5.35 €/kg when transporting hydrogen to the shore by pipeline, which was lower than gas liquefaction. This cost could be reduced to 2.17 €/kg if the European Union supported the hydrogen production project. In another study, the hydrogen produced from the wind/H2 system was coupled with a methane production unit (Ishaq and Dincer 2020). This combination generated 3.4 g/s and 52.25 g/s of hydrogen and methane, respectively.
Moreover, the results showed that while carbon dioxide emissions were reduced by 2999 tons/year, the system efficiency and exergy were 42.3% and 40.5%, respectively. As a result, the wind/H2 system had six times higher energy and exergy efficiencies than the OTEC/H2 system (Ishaq and Dincer 2020). Finally, wind and CPVT energy sources were compared on the basis of hydrogen production in Morocco (Khouya 2020). Investigations revealed that the LCOH ranged from 2.36 to 2.66 $/kg for wind energy to 3.17–4.54 $/kg for CPVT. This comparison was convenient because while wind turbines worked day and night, solar worked only during the day.
Hybrid solar and wind hydrogen production system
The potential to find a stand-alone system to produce electricity in remote areas gets more attention day by day. Producing hydrogen from solar and wind energy is stored for electricity production via a fuel cell in case of excess electricity or selling hydrogen directly to the market (Bernal-Agustín and Dufo-López 2010; Nasser et al. 2022b). The main drawback of using wind and solar separately is the high hydrogen production cost compared to other energy sources, as mentioned above. Therefore, combining wind and solar energy to create a hybrid hydrogen production system (WS/H2) might provide a cost-reduction solution (Nasser et al. 2022a). Moreover, this system offers continuous production because it depends on two energy sources to avoid intermittency periods.
The basic concept of the WS/H2 system is illustrated in (Nasser et al. 2022a; Babatunde et al. 2022) and shown in Fig. 7. This system offers excellent potential in electricity production compared to the traditional one because of the combination of solar and wind energy. Additionally, selling hydrogen at 10 €/kg is economically viable in areas with high wind speed. The hybrid can be created by combining PV/H2 and wind/H2 systems (Akyuz et al. 2012; Babatunde et al. 2022). The results demonstrated that the efficiency of PV/H2 and wind/H2 is 7.9–8.5% and 5–14%, respectively, with hydrogen production of 30.4 kg between April and July.
The hydrogen production from the hybrid system is more than wind/H2 and PV/H2 by 26.2% and 127%, respectively (Khalilnejad and Riahy 2014; Huang et al. 2015). Furthermore, wind turbine contribution is more than PV panels because it works all day and PV panels work at noon only. The thermal efficiency of combining a wind farm with CPVT is better than combining it with CSP (Cai et al. 2020).
The efficiency of hydrogen production increased when using the hybrid system by two factors: (I) an increase in electricity input to the electrolyzer and (II) an increase in the water temperature by solar energy before entering the electrolyzer (Huang et al. 2016). Hydrogen production reaches up to 0.51 Kg/h, and it can be used for refueling stations for hydrogen vehicles. The cost reached 12.3 €/kg when the electricity cost 10 c€/KWh (Bernal-Agustín and Dufo-López 2010). In addition, when a battery system is installed with WS/H2 system, the number of electrolyzer stops is reduced, and working hours, efficiency, and lifetime boost (Ursúa et al. 2016). The hybrid system’s utilization factor is higher than the single system (Papadopoulos et al. 2018).
Some studies are done to investigate the uses of this system in real life: for electricity production for 150m2 houses (Devrim and Bilir 2016), providing hydrogen for hydrogen vehicles (Rezaei et al. 2019; van der Roest et al. 2020) and ammonia, and urea production (Armijo and Philibert 2020; Ishaq et al. 2021). The results proved that the proposed system could provide a house in Turkey with the required electricity around the year except for November. Similarly, the system produces 91 Kg/day of hydrogen, providing 91 cars with energy per week in Iran. In Chile and Argentina, ammonia is manufactured directly from the system with 500 $/ton when the hydrogen costs 2 $/Kg.
In Canada, the hybrid system is designed for hydrogen and urea with 518.4 kmol/day and 86.4 kmol/day, respectively (Ishaq et al. 2021). This hybrid system can provide 540 hydrogen-electric vehicles with the required hydrogen at LCOH equal to 8.7 €/kg, which is lower than the end-user cost (10 €/kg) in the Netherlands (van der Roest et al. 2020). This LCOH can be reduced by 20–26% when considering the avoiding cost of CO2 emissions, which is about 3600 tons per year. Additionally, the introduced system provides distilled water to the electrolyzer by the reverse osmosis system powered by renewable energy. Finally, the hybrid system is used not only for hydrogen production but also for space cooling, heating, and desalination, as shown in Fig. 8. The energy and exergy efficiency of the whole system is 61.34% and 47.8%, respectively, with hydrogen production of 239 Kg/h (Sezer et al. 2019). The dissipated heat from a fuel cell is also used to operate a Stirling engine for electricity production (Wang et al. 2021). The results showed that the Stirling engine produces about 1033.7 W representing 9.85% of the output power.
The schematic diagram for a multi-generation hybrid system for hydrogen production, space cooling, heating, and desalination (Sezer et al. 2019)
Economic assessment of green hydrogen production
Electricity plays a vital role in hydrogen production because it is the primary input to electrolyzers that controls hydrogen production costs. Moreover, several factors such as the lifetime, land cost, and construction period influence electricity production’s pricing from renewable energy. Fig. 9a demonstrates the LCOE implications for different energy sources (renewable and nonrenewable) (Shen et al. 2020). This figure shows that although traditional power sources like coal and nuclear have the lowest LCOE, they negatively affect the environment due to green gas emissions. In contrast, while renewable energy sources have higher LCOEs than traditional ones, they are preferred nowadays because the world is trying to convert to zero emission power generation, proposing that its cost will be reduced.
When calculating LCOH, electricity cost directly affects the results (Nasser et al. 2022a, 2022b). Fig. 9b illustrates the LCOH for different techniques (El-Emam and Özcan 2019; Razi and Dincer 2020). As shown in this figure, the production methods that depend on conventional energy sources had lower LCOH than green methods. Notably, although these methods were expensive, they have attracted more attention for being used in constructing a sustainable society. For example, while the LCOH produced from solar and wind energies varied from 3.41 to 16.01 $/kg and 5.27 to 8.01 $/kg, respectively, the LCOH of a hybrid system lay in the midway of the solar and wind.
Climatic conditions also play a crucial role in producing electricity and hydrogen from solar and wind energy due to the dependence of these sources on climate. As a result, the production of each system mentioned in this study is influenced by location changes from one place to another. Fig. 10 illustrates the LCOH gathered from this review for the understudied systems. This figure demonstrates that although the LCOH varied by the country for similar designs, the average cost of hydrogen production for wind energy was lower than that for solar because it worked day and night.
Studies have also reported that the electrolyzer type coupled with similar energy sources changes the LCOH. For example, LCOH from wind energy associated with PEM varies from 5 to 9.37 $/kg (Olateju et al. 2014, 2016; El-Emam and Özcan 2019). However, for AWE, the LCOH ranged from 7.47 to 7.6 $/kg (Greiner et al. 2007). In other studies, the LCOH when SOE is used varies from 6 to 9.2 $/kg and should decline to 2 $/kg by 2050 (Mastropasqua et al. 2020; Khatiwada et al. 2022). Thus, to sum up, the two main parameters that influence the LCOH from renewable sources are (I) weather conditions (e.g., solar radiation and wind speed) and (II) electrolyzer type.
Economic challenges
Green hydrogen production is expensive and may remain so without government support and action. This fact is because in developing countries with a vast supply of natural resources for power generation, minimized hydrogen production costs are observed, accounting for distance and demand. Even in areas with abundant renewable energy resources, electricity represents a massive part of the manufacturing expenses, with electrolyzers and other expenditures being insignificant.
Therefore, authorities must enhance their energy budget to encourage green hydrogen. For example, the savings from reducing fossil fuel subsidies could be used to fund green hydrogen fuel. However, increasing production would need developing hydrogen infrastructure, a massive effort that requires a solid plan and political backing. Therefore, the authorities should collaborate with recognized firms in creating green hydrogen infrastructure to develop a strategic plan for green hydrogen’s success in the market and the expansion of such infrastructure (Agaton et al. 2022).
Conclusion
A clean energy carrier, hydrogen, is expected to significantly influence this millennium by offering an ecologically friendly choice to meet the world’s rising energy needs. Moreover, since current research has focused on developing green methods for shifting toward a viable and cost-effective hydrogen economy to race with fossil fuel hydrogen generation, this study focused on hydrogen production from the green path using wind and solar energy. The conclusions drawn from this review work are as follows:
-
Hydrogen is an alternate energy carrier that can be stored and transferred and has a high calorific value, making it suited to replace fossil fuels.
-
Green production attracts more attention due to its ability to produce hydrogen with zero carbon emissions. However, coupling the energy source with a water electrolyzer is a better production technique.
-
The electrolyzers can be divided into low-temperature electrolyzers (PEM, AWE, and AEM) and high-temperature electrolyzer (SOE). The low-temperature electrolyzers do not need any heat source and only require an electric source. However, a heat source helps to convert water to steam in SOE, including a power source for water decomposition.
-
PEM electrolyzers are the most suitable to be attached to a green production system due to their low-start period. As a result, they can deal with load fluctuations. However, the SOE type is also suitable for dealing with the CSP/H2 system due to the high temperatures produced by CSP.
-
PV/H2 and wind/H2 systems do not need any power cycle for electricity production, so they need minimum maintenance attention and are suitable for installation in arid areas. In contrast, CSP/H2 requires a power cycle, so maintenance cost must be considered.
-
Although green hydrogen production systems are used for multi-generation purposes such as heating, cooling, and water desalination besides hydrogen production, the system’s performance depends on several factors, such as climatic conditions, tracking systems, control systems, and the electrolyzer type.
-
The LCOH of WS/H2 and solar/H2 is relatively similar, but the LCOH of wind/H2 is higher. Therefore, although a hydrogen compression system raises the LCOH due to the increased capital cost, government support should be able to reduce this LCOH.
-
The primary goal of commercializing a green hydrogen production system is to reduce the capital investment of its components and improve the components’ efficiency. These enhancements will enable green hydrogen to compete with other production methods in the global market.
Data availability
Not applicable.
References
Abdelshafy AM, Hassan H, Jurasz J (2018) Optimal design of a grid-connected desalination plant powered by renewable energy resources using a hybrid PSO–GWO approach. Energy Convers Manag 173:331–347. https://doi.org/10.1016/J.ENCONMAN.2018.07.083
Abohamzeh E, Salehi F, Sheikholeslami M et al (2021) Review of hydrogen safety during storage, transmission, and applications processes. J Loss Prev Process Ind 72:104569. https://doi.org/10.1016/j.jlp.2021.104569
Agaton CB, Batac KIT, Reyes EM (2022) Prospects and challenges for green hydrogen production and utilization in the Philippines. Int J Hydrog Energy 47:17859–17870. https://doi.org/10.1016/J.IJHYDENE.2022.04.101
Akikur RK, Saidur R, Ping HW, Ullah KR (2014) Performance analysis of a co-generation system using solar energy and SOFC technology. Energy Convers Manag 79:415–430. https://doi.org/10.1016/j.enconman.2013.12.036
Akyuz E, Oktay Z, Dincer I (2012) Performance investigation of hydrogen production from a hybrid wind-PV system. Int J Hydrog Energy 37:16623–16630. https://doi.org/10.1016/j.ijhydene.2012.02.149
Al Zahrani AA, Dincer I (2016) Design and analysis of a solar tower based integrated system using high temperature electrolyzer for hydrogen production. Int J Hydrog Energy 41:8042–8056. https://doi.org/10.1016/j.ijhydene.2015.12.103
Allouhi A (2020) Management of photovoltaic excess electricity generation via the power to hydrogen concept: a year-round dynamic assessment using artificial neural networks. Int J Hydrog Energy 45:21024–21039. https://doi.org/10.1016/j.ijhydene.2020.05.262
Almutairi K, Hosseini Dehshiri SS, Hosseini Dehshiri SJ et al (2021) A thorough investigation for development of hydrogen projects from wind energy: a case study. Int J Hydrog Energy 46:18795–18815. https://doi.org/10.1016/j.ijhydene.2021.03.061
Armijo J, Philibert C (2020) Flexible production of green hydrogen and ammonia from variable solar and wind energy: case study of Chile and Argentina. Int J Hydrog Energy 45:1541–1558. https://doi.org/10.1016/j.ijhydene.2019.11.028
Arregi A, Amutio M, Lopez G et al (2018) Evaluation of thermochemical routes for hydrogen production from biomass: a review. Energy Convers Manag 165:696–719. https://doi.org/10.1016/J.ENCONMAN.2018.03.089
Atiz A (2020) Comparison of three different solar collectors integrated with geothermal source for electricity and hydrogen production. Int J Hydrog Energy 45:31651–31666. https://doi.org/10.1016/j.ijhydene.2020.08.236
Atiz A, Karakilcik H, Erden M, Karakilcik M (2019) Assessment of electricity and hydrogen production performance of evacuated tube solar collectors. Int J Hydrog Energy 44:14137–14144. https://doi.org/10.1016/j.ijhydene.2018.09.100
Ayodele TRR, Mosetlhe TCC, Yusuff AAA, Ntombela M (2021) Optimal design of wind-powered hydrogen refuelling station for some selected cities of South Africa. Int J Hydrog Energy 46:24919–24930. https://doi.org/10.1016/j.ijhydene.2021.05.059
Babatunde OM, Munda JL, Hamam Y (2022) Hybridized off-grid fuel cell/wind/solar PV /battery for energy generation in a small household: a multi-criteria perspective. Int J Hydrog Energy 47:6437–6452. https://doi.org/10.1016/J.IJHYDENE.2021.12.018
Balta MT, Kizilkan O, Yilmaz F (2016) Energy and exergy analyses of integrated hydrogen production system using high temperature steam electrolysis. Int J Hydrog Energy 41:8032–8041. https://doi.org/10.1016/j.ijhydene.2015.12.211
Bernal-Agustín JL, Dufo-López R (2010) Techno-economical optimization of the production of hydrogen from PV-wind systems connected to the electrical grid. Renew Energy 35:747–758. https://doi.org/10.1016/j.renene.2009.10.004
Bhandari R, Trudewind CA, Zapp P (2014) Life cycle assessment of hydrogen production via electrolysis - a review. J Clean Prod 85:151–163. https://doi.org/10.1016/j.jclepro.2013.07.048
Bhattacharyya R, Misra A, Sandeep KC (2017) Photovoltaic solar energy conversion for hydrogen production by alkaline water electrolysis: conceptual design and analysis. Energy Convers Manag 133:1–13. https://doi.org/10.1016/j.enconman.2016.11.057
Bicer Y, Dincer I (2017) Experimental investigation of a PV-coupled photoelectrochemical hydrogen production system. Int J Hydrog Energy 42:2512–2521. https://doi.org/10.1016/j.ijhydene.2016.02.098
Bilgen E (2001) Solar hydrogen from photovoltaic-electrolyzer systems. Energy Convers Manag 42:1047–1057. https://doi.org/10.1016/S0196-8904(00)00131-X
Burton NA, Padilla RV, Rose A, Habibullah H (2021) Increasing the efficiency of hydrogen production from solar powered water electrolysis. Renew Sust Energ Rev 135:110255. https://doi.org/10.1016/j.rser.2020.110255
Buttler A, Spliethoff H (2018) Current status of water electrolysis for energy storage, grid balancing and sector coupling via power-to-gas and power-to-liquids: a review. Renew Sust Energ Rev 82:2440–2454
Cai D, Bamisile O, Adebayo V et al (2020) Integration of wind turbine with heliostat based CSP/CPVT system for hydrogen production and polygeneration: a thermodynamic comparison. Int J Hydrog Energy. https://doi.org/10.1016/j.ijhydene.2020.11.106
Carmo M, Fritz DL, Mergel J, Stolten D (2013) A comprehensive review on PEM water electrolysis. Int J Hydrog Energy 38:4901–4934
Carton JG, Olabi AG (2010) Wind/hydrogen hybrid systems: opportunity for Ireland’s wind resource to provide consistent sustainable energy supply. Energy 35:4536–4544. https://doi.org/10.1016/j.energy.2010.09.010
Chadegani EA, Sharifishourabi M, Hajiarab F (2018) Comprehensive assessment of a multi-generation system integrated with a desalination system: modeling and analysing. Energy Convers Manag 174:20–32. https://doi.org/10.1016/j.enconman.2018.08.011
Chen X, Liu Q, Xu J et al (2020) Thermodynamic study of a hybrid PEMFC-solar energy multi-generation system combined with SOEC and dual Rankine cycle. Energy Convers Manag 226:113512. https://doi.org/10.1016/j.enconman.2020.113512
Chi J, Yu H (2018) Water electrolysis based on renewable energy for hydrogen production. Cuihua Xuebao/Chin J Catal 39:390–394
Chien FS, Ngo QT, Hsu CC et al (2021) Assessing the capacity of renewable power production for green energy system: a way forward towards zero carbon electrification. Environ Sci Pollut Res 28:65960–65973. https://doi.org/10.1007/S11356-021-15517-7/FIGURES/3
Cho MK, Park HY, Lee HJ et al (2018) Alkaline anion exchange membrane water electrolysis: effects of electrolyte feed method and electrode binder content. J Power Sources 382:22–29. https://doi.org/10.1016/j.jpowsour.2018.02.025
Concha D, Renaudineau H, Hernández MS et al (2021) Evaluation of DCX converters for off-grid photovoltaic-based green hydrogen production. Int J Hydrog Energy 46:19861–19870. https://doi.org/10.1016/j.ijhydene.2021.03.129
Dabar OA, Awaleh MO, Waberi MM, Adan A-BI (2022) Wind resource assessment and techno-economic analysis of wind energy and green hydrogen production in the Republic of Djibouti. Energy Rep 8:8996–9016. https://doi.org/10.1016/J.EGYR.2022.07.013
David M, Ocampo-Martínez C, Sánchez-Peña R (2019) Advances in alkaline water electrolyzers: a review. J Energy Storage 23:392–403. https://doi.org/10.1016/j.est.2019.03.001
Delpisheh M, Haghghi MA, Athari H, Mehrpooya M (2021) Desalinated water and hydrogen generation from seawater via a desalination unit and a low temperature electrolysis using a novel solar-based setup. Int J Hydrog Energy 46:7211–7229. https://doi.org/10.1016/j.ijhydene.2020.11.215
Demirdelen T, Ekinci F, Mert BD et al (2020) Green touch for hydrogen production via alkaline electrolysis: the semi-flexible PV panels mounted wind turbine design, production and performance analysis. Int J Hydrog Energy 45:10680–10695. https://doi.org/10.1016/j.ijhydene.2020.02.007
Devrim Y, Bilir L (2016) Performance investigation of a wind turbine–solar photovoltaic panels–fuel cell hybrid system installed at İncek region – Ankara, Turkey. Energy Convers Manag 126:759–766. https://doi.org/10.1016/j.enconman.2016.08.062
Dincer I, Zamfirescu C (2011) Sustainable energy systems and applications. Springer Science & Business Media
Dincer I, Acar C (2014) Review and evaluation of hydrogen production methods for better sustainability. Int J Hydrog Energy 40:11094–11111. https://doi.org/10.1016/j.ijhydene.2014.12.035
Eldesoukey A, Hassan H (2019) 3D model of thermoelectric generator (TEG) case study: effect of flow regime on the TEG performance. Energy Convers Manag 180:231–239. https://doi.org/10.1016/j.enconman.2018.10.104
El-Emam RS, Özcan H (2019) Comprehensive review on the techno-economics of sustainable large-scale clean hydrogen production. J Clean Prod 220:593–609. https://doi.org/10.1016/j.jclepro.2019.01.309
Elghamry R, Hassan H, Hawwash AA (2019) A parametric study on the impact of integrating solar cell panel at building envelope on its power, energy consumption, comfort conditions, and CO2 emissions. J Clean Prod 249:119374. https://doi.org/10.1016/J.JCLEPRO.2019.119374
Elsevier (2022) Scopus database. https://www.scopus.com/standard/marketing.uri. Accessed 23 Jul 2022
Erzen S, Açıkkalp E, Hepbasli A (2020) Performance analysis of a solar–hydrogen driven multigeneration system. Energy Rep 6:403–408. https://doi.org/10.1016/J.EGYR.2019.08.080
Faid AY, Barnett AO, Seland F, Sunde S (2018) Highly active nickel-based catalyst for hydrogen evolution in anion exchange membrane electrolysis. Catalysts 8:614. https://doi.org/10.3390/catal8120614
Fan L, Tu Z, Chan SH (2021) Recent development of hydrogen and fuel cell technologies: a review. Energy Rep 7:8421–8446. https://doi.org/10.1016/J.EGYR.2021.08.003
Fang R, Liang Y (2019) Control strategy of electrolyzer in a wind-hydrogen system considering the constraints of switching times. Int J Hydrog Energy 44:25104–25111. https://doi.org/10.1016/j.ijhydene.2019.03.033
Ferrari ML, Rivarolo M, Massardo AF (2016) Hydrogen production system from photovoltaic panels: experimental characterization and size optimization. Energy Convers Manag 116:194–202. https://doi.org/10.1016/j.enconman.2016.02.081
Fopah-Lele A, Kabore-Kere A, Tamba JG, Yaya-Nadjo I (2021) Solar electricity storage through green hydrogen production: a case study. Int J Energy Res:1–15. https://doi.org/10.1002/er.6630
Franco BA, Baptista P, Neto RC, Ganilha S (2021) Assessment of offloading pathways for wind-powered offshore hydrogen production: energy and economic analysis. Appl Energy 286:116553. https://doi.org/10.1016/j.apenergy.2021.116553
Gado M, Tamer M, Ookawara S et al (2022) Performance assessment of photovoltaic/thermal (PVT) hybrid adsorption-vapor compression refrigeration system. J Energy Syst 6:209–220. https://doi.org/10.30521/jes.1002871
Ganeshan IS, Manikandan VVS, Ram Sundhar V et al (2016) Regulated hydrogen production using solar powered electrolyser. Int J Hydrog Energy 41:10322–10326. https://doi.org/10.1016/j.ijhydene.2015.05.048
Genç MS, Çelik M, Karasu I (2012) A review on wind energy and wind-hydrogen production in Turkey: a case study of hydrogen production via electrolysis system supplied by wind energy conversion system in Central Anatolian Turkey. Renew Sust Energ Rev 16:6631–6646
Geovanni HG, Orlando LD, Rafael PD et al (2010) Analysis of the current methods used to size a wind/hydrogen/fuel cell-integrated system: a new perspective. Int J Energy Res 34:1042–1051. https://doi.org/10.1002/er.1626
Gibson TL, Kelly NA (2008) Optimization of solar powered hydrogen production using photovoltaic electrolysis devices. Int J Hydrog Energy 33:5931–5940. https://doi.org/10.1016/j.ijhydene.2008.05.106
Gibson TL, Kelly NA (2010) Predicting efficiency of solar powered hydrogen generation using photovoltaic-electrolysis devices. Int J Hydrog Energy 35:900–911. https://doi.org/10.1016/j.ijhydene.2009.11.074
Gill EZ, Ratlamwala TAH, Hussain G, Alkahtani M (2021) Energy, exergy, exergo-economic and exergo-environmental analyses of solar based hydrogen generation system. Int J Hydrog Energy 46:29049–29064. https://doi.org/10.1016/j.ijhydene.2020.07.100
Greiner CJ, KorpÅs M, Holen AT (2007) A Norwegian case study on the production of hydrogen from wind power. Int J Hydrog Energy 32:1500–1507. https://doi.org/10.1016/j.ijhydene.2006.10.030
Grimm A, de Jong WA, Kramer GJ (2020) Renewable hydrogen production: a techno-economic comparison of photoelectrochemical cells and photovoltaic-electrolysis. Int J Hydrog Energy 45:22545–22555. https://doi.org/10.1016/j.ijhydene.2020.06.092
Grüger F, Hoch O, Hartmann J et al (2019) Optimized electrolyzer operation: employing forecasts of wind energy availability, hydrogen demand, and electricity prices. Int J Hydrog Energy 4:4387–4397. https://doi.org/10.1016/j.ijhydene.2018.07.165
Gupta RB (2009) Hydrogen Fuel. CRC Press, Boca Raton
Gutiérrez-Martín F, Amodio L, Pagano M (2020) Hydrogen production by water electrolysis and off-grid solar PV. Int J Hydrog Energy. https://doi.org/10.1016/j.ijhydene.2020.09.098
Hacker V, Fankhauser R, Faleschini G et al (2000) Hydrogen production by steam–iron process. J Power Sources 86:531–535. https://doi.org/10.1016/S0378-7753(99)00458-9
Haider SA, Sajid M, Iqbal S (2021) Forecasting hydrogen production potential in Islamabad from solar energy using water electrolysis. Int J Hydrog Energy 46:1671–1681. https://doi.org/10.1016/j.ijhydene.2020.10.059
Hassan IA, Ramadan HS, Saleh MA, Hissel D (2021) Hydrogen storage technologies for stationary and mobile applications: review, analysis and perspectives. Renew Sust Energ Rev 149:111311. https://doi.org/10.1016/J.RSER.2021.111311
Hassan AA, Elwardany AE, Ookawara S, Hassan H (2022) Energy, exergy, economic and environmental (4E) assessment of hybrid solar system powering adsorption-parallel/series ORC multigeneration system. Process Saf Environ Prot 164:761–780. https://doi.org/10.1016/J.PSEP.2022.06.024
He W, Namar MM, Li Z et al (2020) Thermodynamic analysis of a solar-driven high-temperature steam electrolyzer for clean hydrogen production. Appl Therm Eng 172:115152. https://doi.org/10.1016/j.applthermaleng.2020.115152
Herwartz S, Pagenkopf J, Streuling C (2021) Sector coupling potential of wind-based hydrogen production and fuel cell train operation in regional rail transport in Berlin and Brandenburg. Int J Hydrog Energy. https://doi.org/10.1016/j.ijhydene.2020.11.242
Holladay JD, Hu J, King DL, Wang Y (2009) An overview of hydrogen production technologies. Catal Today 139:244–260. https://doi.org/10.1016/j.cattod.2008.08.039
Hosseini SE, Butler B (2021) Design and analysis of a hybrid concentrated photovoltaic thermal system integrated with an organic Rankine cycle for hydrogen production. J Therm Anal Calorim 144:763–778. https://doi.org/10.1007/s10973-020-09556-4
Hosseini SE, Wahid MA (2020) Hydrogen from solar energy, a clean energy carrier from a sustainable source of energy. Int J Energy Res 44:4110–4131. https://doi.org/10.1002/er.4930
Houaijia A, Roeb M, Monnerie N, Sattler C (2015) Solar power tower as heat and electricity source for a solid oxide electrolyzer: a case study. Int J Energy Res 39:1120–1130. https://doi.org/10.1002/er.3316
Huang Q, Shi Y, Wang Y et al (2015) Multi-turbine wind-solar hybrid system. Renew. Energy 76:401–407. https://doi.org/10.1016/j.renene.2014.11.060
Huang PH, Kuo JK, Wu ZD (2016) Applying small wind turbines and a photovoltaic system to facilitate electrolysis hydrogen production. Int J Hydrog Energy 41:8514–8524. https://doi.org/10.1016/j.ijhydene.2016.02.051
IEA (2020) Projected Costs of Generating Electricity 2020 – Analysis - IEA. https://www.iea.org/reports/projected-costs-of-generating-electricity-2020. Accessed 26 Jul 2022
Iqbal MT (2003) Simulation of a small wind fuel cell hybrid energy system. Renew Energy 28:511–522. https://doi.org/10.1016/S0960-1481(02)00070-8
Iqbal W, Yumei H, Abbas Q et al (2019) Assessment of wind energy potential for the production of renewable hydrogen in Sindh Province of Pakistan. Processes 7:196. https://doi.org/10.3390/pr7040196
IRENA2022 Workbook: IRENA_Electrolysers_Patents_Insights. https://public.tableau.com/views/IRENA_Electrolysers_Patents_Insights/Electrolysers_patent_insight?:showVizHome=no&embed=yes. Accessed 29 Jul 2022
Ishaq H, Dincer I (2020) Evaluation of a wind energy based system for co-generation of hydrogen and methanol production. Int J Hydrog Energy 45:15869–15877. https://doi.org/10.1016/j.ijhydene.2020.01.037
Ishaq H, Siddiqui O, Chehade G, Dincer I (2021) A solar and wind driven energy system for hydrogen and urea production with CO2 capturing. Int J Hydrog Energy 46:4749–4760. https://doi.org/10.1016/j.ijhydene.2020.01.208
Ismail TM, Ramzy K, Elnaghi BE et al (2019) Using MATLAB to model and simulate a photovoltaic system to produce hydrogen. Energy Convers Manag 185:101–129. https://doi.org/10.1016/j.enconman.2019.01.108
Joshi AS, Dincer I, Reddy BV (2010) Exergetic assessment of solar hydrogen production methods. Int J Hydrog Energy 35:4901–4908. https://doi.org/10.1016/j.ijhydene.2009.09.067
Joshi AS, Dincer I, Reddy BV (2011) Solar hydrogen production: a comparative performance assessment. Int J Hydrog Energy 36:11246–11257. https://doi.org/10.1016/j.ijhydene.2010.11.122
Khalilnejad A, Riahy GH (2014) A hybrid wind-PV system performance investigation for the purpose of maximum hydrogen production and storage using advanced alkaline electrolyzer. Energy Convers Manag 80:398–406. https://doi.org/10.1016/j.enconman.2014.01.040
Khan AA, Khan SU, Ali MAS et al (2022) Identifying impact of international trade and renewable energy consumption on environmental quality improvement and their role in global warming. Environ Sci Pollut Res 29:33935–33944. https://doi.org/10.1007/S11356-022-18574-8/TABLES/7
Khatiwada D, Vasudevan RA, Santos BH (2022) Decarbonization of natural gas systems in the EU – costs, barriers, and constraints of hydrogen production with a case study in Portugal. Renew Sust Energ Rev 168:112775. https://doi.org/10.1016/J.RSER.2022.112775
Khelfaoui N, Djafour A, Ghenai C et al (2020) Experimental investigation of solar hydrogen production PV/PEM electrolyser performance in the Algerian Sahara regions. Int J Hydrog Energy 46:30524–30538. https://doi.org/10.1016/j.ijhydene.2020.11.193
Khouya A (2020) Levelized costs of energy and hydrogen of wind farms and concentrated photovoltaic thermal systems. A case study in Morocco. Int J Hydrog Energy 45:31632–31650. https://doi.org/10.1016/j.ijhydene.2020.08.240
Khouya A (2021) Hydrogen production costs of a polymer electrolyte membrane electrolysis powered by a renewable hybrid system. Int J Hydrog Energy 46:14005–14023. https://doi.org/10.1016/j.ijhydene.2021.01.213
Koleva M, Guerra OJ, Eichman J et al (2021) Optimal design of solar-driven electrolytic hydrogen production systems within electricity markets. J Power Sources 483:229183. https://doi.org/10.1016/j.jpowsour.2020.229183
Koumi Ngoh S, Ayina Ohandja LM, Kemajou A, Monkam L (2014) Design and simulation of hybrid solar high-temperature hydrogen production system using both solar photovoltaic and thermal energy. Sustain Energy Technol Assess 7:279–293. https://doi.org/10.1016/j.seta.2014.05.002
Kovács KL, Maróti G, Rákhely G (2006) A novel approach for biohydrogen production. Int J Hydrog Energy 31:1460–1468. https://doi.org/10.1016/j.ijhydene.2006.06.011
Lagorse J, Simões MG, Miraoui A, Costerg P (2008) Energy cost analysis of a solar-hydrogen hybrid energy system for stand-alone applications. Int J Hydrog Energy 33:2871–2879. https://doi.org/10.1016/j.ijhydene.2008.03.054
Lahoussine Ouali HA, Moussaoui MA, Mezrhab A (2020) Hydrogen production from two commercial dish/Stirling systems compared to the photovoltaic system-case study: Eastern Morocco. Appl Sol Energy (English Transl Geliotekhnika) 56:466–476. https://doi.org/10.3103/S0003701X20060080
Li C, Baek J-BB (2021) The promise of hydrogen production from alkaline anion exchange membrane electrolyzers. Nano Energy 87:106162
Li G, Li J, Yang R, Chen X (2022a) Performance analysis of a hybrid hydrogen production system in the integrations of PV/T power generation electrolytic water and photothermal cooperative reaction. Appl Energy 323:119625. https://doi.org/10.1016/J.APENERGY.2022.119625
Li H, Cao X, Liu Y et al (2022b) Safety of hydrogen storage and transportation: an overview on mechanisms, techniques, and challenges. Energy Rep 8:6258–6269. https://doi.org/10.1016/J.EGYR.2022.04.067
Lim T, Kim SK (2022) Non-precious hydrogen evolution reaction catalysts: stepping forward to practical polymer electrolyte membrane-based zero-gap water electrolyzers. Chem Eng J 433:133681. https://doi.org/10.1016/J.CEJ.2021.133681
Lin H, Wu Q, Chen X et al (2021) Economic and technological feasibility of using power-to-hydrogen technology under higher wind penetration in China. Renew Energy 173:569–580. https://doi.org/10.1016/j.renene.2021.04.015
Liu J, Abbas Q, Alharthi M et al (2021) Managerial policy and economic analysis of wind-generated renewable hydrogen for light-duty vehicles: green solution of energy crises. Environ Sci Pollut Res 28:10642–10653. https://doi.org/10.1007/S11356-020-11018-1/TABLES/9
Luo Z, Wang X, Wen H, Pei A (2022) Hydrogen production from offshore wind power in South China. Int J Hydrog Energy. https://doi.org/10.1016/J.IJHYDENE.2022.03.162
Marefati M, Mehrpooya M (2019) Introducing a hybrid photovoltaic solar, proton exchange membrane fuel cell and thermoelectric device system. Sustain Energy Technol Assess 36:100550. https://doi.org/10.1016/j.seta.2019.100550
Marefati M, Mehrpooya M, Mousavi SA (2019) Introducing an integrated SOFC, linear Fresnel solar field, Stirling engine and steam turbine combined cooling, heating and power process. Int J Hydrog Energy 44:30256–30279. https://doi.org/10.1016/j.ijhydene.2019.09.074
Martinez-Burgos WJ, de Souza CE, Pedroni Medeiros AB et al (2021) Hydrogen: current advances and patented technologies of its renewable production. J Clean Prod 286:124970. https://doi.org/10.1016/J.JCLEPRO.2020.124970
Mastropasqua L, Pecenati I, Giostri A, Campanari S (2020) Solar hydrogen production: techno-economic analysis of a parabolic dish-supported high-temperature electrolysis system. Appl Energy 261:114392. https://doi.org/10.1016/j.apenergy.2019.114392
Matute G, Yusta JM, Beyza J, Monteiro C (2022) Optimal dispatch model for PV-electrolysis plants in self-consumption regime to produce green hydrogen: a Spanish case study. Int J Hydrog Energy 47:25202–25213. https://doi.org/10.1016/J.IJHYDENE.2022.05.270
Mert BD, Ekinci F, Demirdelen T (2019) Effect of partial shading conditions on off-grid solar PV/hydrogen production in high solar energy index regions. Int J Hydrog Energy 44:27713–27725. https://doi.org/10.1016/j.ijhydene.2019.09.011
Mokhtara C, Negrou B, Settou N et al (2020) Design optimization of grid-connected PV-hydrogen for energy prosumers considering sector-coupling paradigm: case study of a university building in Algeria. Int J Hydrog Energy. https://doi.org/10.1016/j.ijhydene.2020.10.069
Moradi Nafchi F, Baniasadi E, Afshari E, Javani N (2018) Performance assessment of a solar hydrogen and electricity production plant using high temperature PEM electrolyzer and energy storage. Int J Hydrog Energy 43:5820–5831. https://doi.org/10.1016/j.ijhydene.2017.09.058
Motealleh B, Liu Z, Masel RI et al (2021) Next-generation anion exchange membrane water electrolyzers operating for commercially relevant lifetimes. Int J Hydrog Energy 46:3379–3386. https://doi.org/10.1016/j.ijhydene.2020.10.244
Muhammad-Bashir S, Al-Oufi M, Al-Hakami M et al (2020) Comparison between the performance of high concentrated and non-concentrated PV-cells for hydrogen production using PEM water electrolyzers. Sol Energy 205:461–464. https://doi.org/10.1016/j.solener.2020.05.077
Muyeen SM, Takahashi R, Tamura J (2011) Electrolyzer switching strategy for hydrogen generation from variable speed wind generator. Electr Power Syst Res 81:1171–1179. https://doi.org/10.1016/j.epsr.2011.01.005
Nadaleti WC, Borges dos Santos G, Lourenço VA (2019) The potential and economic viability of hydrogen production from the use of hydroelectric and wind farms surplus energy in Brazil: a national and pioneering analysis. Int J Hydrog Energy 5:0–11. https://doi.org/10.1016/j.ijhydene.2019.08.199
Naseri A, Bidi M, Ahmadi MH, Saidur R (2017) Exergy analysis of a hydrogen and water production process by a solar-driven transcritical CO2 power cycle with Stirling engine. J Clean Prod 158:165–181. https://doi.org/10.1016/j.jclepro.2017.05.005
Nasser M, Megahed TF, Ookawara S, Hassan H (2022a) Performance evaluation of PV panels/wind turbines hybrid system for green hydrogen generation and storage: energy, exergy, economic, and enviroeconomic. Energy Convers Manag 267:115870. https://doi.org/10.1016/j.enconman.2022.115870
Nasser M, Megahed TF, Ookawara S, Hassan H (2022b) Techno-economic assessment of clean hydrogen production and storage using hybrid renewable energy system of PV/wind under different climatic conditions. Sustain Energy Technol Assess 52:102195. https://doi.org/10.1016/J.SETA.2022.102195
Ntziachristos L, Kouridis C, Samaras Z, Pattas K (2005) A wind-power fuel-cell hybrid system study on the non-interconnected Aegean islands grid. Renew Energy 30:1471–1487. https://doi.org/10.1016/j.renene.2004.11.007
Okundamiya MS (2020) Size optimization of a hybrid photovoltaic/fuel cell grid connected power system including hydrogen storage. Int J Hydrog Energy. https://doi.org/10.1016/j.ijhydene.2020.11.185
Olateju B, Monds J, Kumar A (2014) Large scale hydrogen production from wind energy for the upgrading of bitumen from oil sands. Appl Energy 118:48–56. https://doi.org/10.1016/j.apenergy.2013.12.013
Olateju B, Kumar A, Secanell M (2016) A techno-economic assessment of large scale wind-hydrogen production with energy storage in Western Canada. Int J Hydrog Energy 41:8755–8776. https://doi.org/10.1016/j.ijhydene.2016.03.177
Ozturk M, Dincer I (2013) Thermodynamic analysis of a solar-based multi-generation system with hydrogen production. Appl Therm Eng 51:1235–1244. https://doi.org/10.1016/j.applthermaleng.2012.11.042
Papadopoulos V, Desmet J, Knockaert J, Develder C (2018) Improving the utilization factor of a PEM electrolyzer powered by a 15 MW PV park by combining wind power and battery storage – feasibility study. Int J Hydrog Energy 43:16468–16478. https://doi.org/10.1016/j.ijhydene.2018.07.069
Pathak AK, Kothari R, Tyagi V, Anand S (2020) Integrated approach for textile industry wastewater for efficient hydrogen production and treatment through solar PV electrolysis. Int J Hydrog Energy 45:25768–25782. https://doi.org/10.1016/j.ijhydene.2020.03.079
Paul B, Andrews J (2008) Optimal coupling of PV arrays to PEM electrolysers in solar-hydrogen systems for remote area power supply. Int J Hydrog Energy 33:490–498. https://doi.org/10.1016/j.ijhydene.2007.10.040
Pereira CA, Coelho PM, Fernandes JF, Gomes MH (2017) Study of an energy mix for the production of hydrogen. Int J Hydrog Energy 42:1375–1382. https://doi.org/10.1016/j.ijhydene.2016.07.182
Privitera SMS, Muller M, Zwaygardt W et al (2020) Highly efficient solar hydrogen production through the use of bifacial photovoltaics and membrane electrolysis. J Power Sources 473:228619. https://doi.org/10.1016/j.jpowsour.2020.228619
Puig-samper G, Bargiacchi E, Iribarren D, Dufour J (2022) Assessing the prospective environmental performance of hydrogen from high-temperature electrolysis coupled with concentrated solar power CSP. Renew Energy 196:1258–1268. https://doi.org/10.1016/j.renene.2022.07.066
Puranen P, Kosonen A, Ahola J (2021) Technical feasibility evaluation of a solar PV based off-grid domestic energy system with battery and hydrogen energy storage in northern climates. Sol Energy 213:246–259. https://doi.org/10.1016/j.solener.2020.10.089
Qiu Y, Li Q, Pan Y et al (2019) A scenario generation method based on the mixture vine copula and its application in the power system with wind/hydrogen production. Int J Hydrog Energy 4:5162–5170. https://doi.org/10.1016/j.ijhydene.2018.09.179
Qureshy AMMI, Dincer I (2020) A new integrated renewable energy system for clean electricity and hydrogen fuel production. Int J Hydrog Energy 45:20944–20955. https://doi.org/10.1016/j.ijhydene.2020.04.218
Rahim AHA, Tijani AS, Fadhlullah M et al (2015) Optimization of direct coupling solar PV panel and advanced alkaline electrolyzer system. In: Energy Procedia. Elsevier Ltd, Oxford, pp 204–211
Ratlamwala TAH, Gadalla MA, Dincer I (2011) Performance assessment of an integrated PV/T and triple effect cooling system for hydrogen and cooling production. Int J Hydrog Energy 36:11282–11291. https://doi.org/10.1016/j.ijhydene.2010.11.121
Razi F, Dincer I (2020) A critical evaluation of potential routes of solar hydrogen production for sustainable development. J Clean Prod 264:121582. https://doi.org/10.1016/j.jclepro.2020.121582
Rezaei M, Mostafaeipour A, Qolipour M, Momeni M (2019) Energy supply for water electrolysis systems using wind and solar energy to produce hydrogen: a case study of Iran. Front Energy 13:539–550. https://doi.org/10.1007/s11708-019-0635-x
Rezaei M, Naghdi-Khozani N, Jafari N (2020) Wind energy utilization for hydrogen production in an underdeveloped country: an economic investigation. Renew Energy 147:1044–1057. https://doi.org/10.1016/j.renene.2019.09.079
Rezaei M, Khalilpour KR, Mohamed MA (2021) Co-production of electricity and hydrogen from wind: a comprehensive scenario-based techno-economic analysis. Int J Hydrog Energy 46:18242–18256. https://doi.org/10.1016/j.ijhydene.2021.03.004
Richards BS, Conibeer GJ (2007) A comparison of hydrogen storage technologies for solar-powered stand-alone power supplies: a photovoltaic system sizing approach. Int J Hydrog Energy 32:2712–2718. https://doi.org/10.1016/j.ijhydene.2006.09.013
Saadi A, Becherif M, Ramadan HS (2016) Hydrogen production horizon using solar energy in Biskra, Algeria. Int J Hydrog Energy 41:21899–21912. https://doi.org/10.1016/j.ijhydene.2016.08.224
Saenz-Aguirre A, Fernandez-Gamiz U, Zulueta E et al (2020) Flow control based 5 MW wind turbine enhanced energy production for hydrogen generation cost reduction. Int J Hydrog Energy 1–13. https://doi.org/10.1016/j.ijhydene.2020.01.022
Sangeetha M, Manigandan S, Ashok B et al (2021) Experimental investigation of nanofluid based photovoltaic thermal (PV/T) system for superior electrical efficiency and hydrogen production. Fuel 286:119422. https://doi.org/10.1016/j.fuel.2020.119422
Sanz-Bermejo J, Gallardo-Natividad V, González-Aguilar J, Romero M (2014a) Coupling of a solid-oxide cell unit and a linear fresnel reflector field for grid management. In: Energy Procedia. Elsevier Ltd, Oxford, pp 706–715
Sanz-Bermejo J, Muñoz-Antón J, Gonzalez-Aguilar J, Romero M (2014b) Optimal integration of a solid-oxide electrolyser cell into a direct steam generation solar tower plant for zero-emission hydrogen production. Appl Energy 131:238–247
Sarrias-Mena R, Fernández-Ramírez LM, García-Vázquez CA, Jurado F (2015) Electrolyzer models for hydrogen production from wind energy systems. Int J Hydrog Energy 40:2927–2938. https://doi.org/10.1016/j.ijhydene.2014.12.125
Saxena RC, Seal D, Kumar S, Goyal HB (2008) Thermo-chemical routes for hydrogen rich gas from biomass: a review. Renew Sust Energ Rev 12:1909–1927. https://doi.org/10.1016/J.RSER.2007.03.005
Sedaghat A, Mostafaeipour A, Rezaei M et al (2020) A new semi-empirical wind turbine capacity factor for maximizing annual electricity and hydrogen production. Int J Hydrog Energy 45:15888–15903. https://doi.org/10.1016/j.ijhydene.2020.04.028
Sen O, Faruk Guler O, Yilmaz C, Kanoglu M (2021) Thermodynamic modeling and analysis of a solar and geothermal assisted multi-generation energy system. Energy Convers Manag 239:114186. https://doi.org/10.1016/j.enconman.2021.114186
Senthilraja S, Gangadevi R, Marimuthu R, Baskaran M (2020) Performance evaluation of water and air based PVT solar collector for hydrogen production application. Int J Hydrog Energy 45:7498–7507. https://doi.org/10.1016/j.ijhydene.2019.02.223
Şevik S (2022) Techno-economic evaluation of a grid-connected PV-trigeneration-hydrogen production hybrid system on a university campus. Int J Hydrog Energy 47:23935–23956. https://doi.org/10.1016/J.IJHYDENE.2022.05.193
Seyyedi SM, Hashemi-Tilehnoee M, Sharifpur M (2022) Thermoeconomic analysis of a solar-driven hydrogen production system with proton exchange membrane water electrolysis unit. Therm Sci Eng Prog 30:101274. https://doi.org/10.1016/j.tsep.2022.101274
Sezer N, Biçer Y, Koç M (2019) Design and analysis of an integrated concentrated solar and wind energy system with storage. Int J Energy Res 43:3263–3283. https://doi.org/10.1002/er.4456
Shaner MR, Atwater HA, Lewis NS, McFarland EW (2016) A comparative technoeconomic analysis of renewable hydrogen production using solar energy. Energy Environ Sci 9:2354–2371. https://doi.org/10.1039/c5ee02573g
Shen W, Chen X, Qiu J et al (2020) A comprehensive review of variable renewable energy levelized cost of electricity. Renew Sust Energ Rev 133:110301. https://doi.org/10.1016/j.rser.2020.110301
Shen Y, Li X, Wang N et al (2021) Introducing and investigation of a pumped hydro-compressed air storage based on wind turbine and alkaline fuel cell and electrolyzer. Sustain Energy Technol Assess 47:101378. https://doi.org/10.1016/j.seta.2021.101378
Sherif SA, Barbir F, Veziroglu TN (2005) Wind energy and the hydrogen economy-review of the technology. Sol Energy 78:647–660. https://doi.org/10.1016/j.solener.2005.01.002
Siddiqui O, Dincer I (2020) Design and transient analyses of a new renewable energy system for multigeneration of power, heat, hydrogen and ammonia. J Clean Prod 270:122502. https://doi.org/10.1016/J.JCLEPRO.2020.122502
Singla MK, Nijhawan P, Oberoi AS (2021) Hydrogen fuel and fuel cell technology for cleaner future: a review. Environ Sci Pollut Res 28:15607–15626. https://doi.org/10.1007/S11356-020-12231-8/FIGURES/13
Soliman AMA, Hassan H (2019) Effect of heat spreader size, microchannel con figuration and nanoparticles on the performance of PV-heat spreader-microchannels system. Sol Energy 182:286–297. https://doi.org/10.1016/j.solener.2019.02.059
Soliman AMA, Hassan H, Ookawara S (2019) An experimental study of the performance of the solar cell with heat sink cooling system. Energy Procedia 162:127–135. https://doi.org/10.1016/j.egypro.2019.04.014
Steinfeld A (2005) Solar thermochemical production of hydrogen - a review. Sol Energy 78:603–615. https://doi.org/10.1016/j.solener.2003.12.012
Sun Z, Wang J, Dai Y, Wang J (2012) Exergy analysis and optimization of a hydrogen production process by a solar-liquefied natural gas hybrid driven transcritical CO2 power cycle. Int J Hydrog Energy 37:18731–18739. https://doi.org/10.1016/j.ijhydene.2012.08.028
Tebibel H (2017) Off grid PV system for hydrogen production using PEM methanol electrolysis and an optimal management strategy. Int J Hydrog Energy 42:19432–19445. https://doi.org/10.1016/j.ijhydene.2017.05.205
Temiz M, Dincer I (2021) Concentrated solar driven thermochemical hydrogen production plant with thermal energy storage and geothermal systems. Energy 219:119554. https://doi.org/10.1016/j.energy.2020.119554
Temiz M, Dincer I (2022) A unique bifacial PV and hydrogen-based cleaner energy system with heat recovery for data centers. Appl Therm Eng 206:118102. https://doi.org/10.1016/J.APPLTHERMALENG.2022.118102
Touili S, Alami Merrouni A, Azouzoute A et al (2018) A technical and economical assessment of hydrogen production potential from solar energy in Morocco. Int J Hydrog Energy 43:22777–22796. https://doi.org/10.1016/j.ijhydene.2018.10.136
Touili S, Alami Merrouni A, El Hassouani Y et al (2020) Analysis of the yield and production cost of large-scale electrolytic hydrogen from different solar technologies and under several Moroccan climate zones. Int J Hydrog Energy 45:26785–26799. https://doi.org/10.1016/j.ijhydene.2020.07.118
Tukenmez N, Yilmaz F, Ozturk M (2021) A thermal performance evaluation of a new integrated gas turbine-based multigeneration plant with hydrogen and ammonia production. Int J Hydrog Energy 46:29012–29026. https://doi.org/10.1016/J.IJHYDENE.2020.11.054
Ulleberg Ø, Nakken T, Eté A (2010) The wind/hydrogen demonstration system at Utsira in Norway: evaluation of system performance using operational data and updated hydrogen energy system modeling tools. Int J Hydrog Energy 35:1841–1852. https://doi.org/10.1016/j.ijhydene.2009.10.077
Ursúa A, Barrios EL, Pascual J et al (2016) Integration of commercial alkaline water electrolysers with renewable energies: limitations and improvements. Int J Hydrog Energy 41:12852–12861. https://doi.org/10.1016/j.ijhydene.2016.06.071
Valdés R, Rodríguez LR, Lucio JH (2012) Procedure for optimal design of hydrogen production plants with reserve storage and a stand-alone photovoltaic power system. Int J Hydrog Energy 37:4018–4025. https://doi.org/10.1016/j.ijhydene.2011.11.133
van der Roest E, Snip L, Fens T, van Wijk A (2020) Introducing power-to-H3: combining renewable electricity with heat, water and hydrogen production and storage in a neighbourhood. Appl Energy 257:114024. https://doi.org/10.1016/j.apenergy.2019.114024
Vidueira JM, Contreras A, Veziroglu TN (2003) PV autonomous installation to produce hydrogen via electrolysis, and its use in FC buses. Int J Hydrog Energy 28:927–937. https://doi.org/10.1016/S0360-3199(02)00191-X
Wang Z, Zhang X, Rezazadeh A (2021) Hydrogen fuel and electricity generation from a new hybrid energy system based on wind and solar energies and alkaline fuel cell. Energy Rep 7:2594–2604. https://doi.org/10.1016/j.egyr.2021.04.060
Wu B, Zhai B, Mu H et al (2021) (2021) Evaluating an economic application of renewable generated hydrogen: a way forward for green economic performance and policy measures. Environ Sci Pollut Res 2910(29):15144–15158. https://doi.org/10.1007/S11356-021-16770-6
Xiao P, Hu W, Xu X et al (2020) Optimal operation of a wind-electrolytic hydrogen storage system in the electricity/hydrogen markets. Int J Hydrog Energy 45:24412–24423. https://doi.org/10.1016/j.ijhydene.2020.06.302
Yan XL, Hino R (2018) Nuclear hydrogen production handbook, 1st edn. CRC Press, Boca Raton
Yilmaz F, Ozturk M, Selbas R (2018) Thermodynamic performance assessment of ocean thermal energy conversion based hydrogen production and liquefaction process. Int J Hydrog Energy 43:10626–10636. https://doi.org/10.1016/j.ijhydene.2018.02.021
Younas M, Shafique S, Hafeez A et al (2022) An overview of hydrogen production: current status, potential, and challenges. Fuel 316:123317. https://doi.org/10.1016/J.FUEL.2022.123317
Zafar S, Dincer I (2014) Thermodynamic analysis of a combined PV/T-fuel cell system for power, heat, fresh water and hydrogen production. Int J Hydrog Energy 39:9962–9972. https://doi.org/10.1016/j.ijhydene.2014.04.127
Zayed ME, Zhao J, Elsheikh AH et al (2021) A comprehensive review on dish/Stirling concentrated solar power systems: design, optical and geometrical analyses, thermal performance assessment, and applications. J Clean Prod 283:124664. https://doi.org/10.1016/j.jclepro.2020.124664
Zhang Y, Wei W (2020) Model construction and energy management system of lithium battery, PV generator, hydrogen production unit and fuel cell in islanded AC microgrid. Int J Hydrog Energy 45:16381–16397. https://doi.org/10.1016/j.ijhydene.2020.04.155
Zhang XR, Yamaguchi H, Uneno D et al (2006) Analysis of a novel solar energy-powered Rankine cycle for combined power and heat generation using supercritical carbon dioxide. Renew Energy 31:1839–1854. https://doi.org/10.1016/j.renene.2005.09.024
Zhang F, Zhao P, Niu M, Maddy J (2016) The survey of key technologies in hydrogen energy storage. Int J Hydrog Energy 41:14535–14552. https://doi.org/10.1016/j.ijhydene.2016.05.293
Zhou T, Francois B (2009) Modeling and control design of hydrogen production process for an active hydrogen/wind hybrid power system. Int J Hydrog Energy 34:21–30. https://doi.org/10.1016/j.ijhydene.2008.10.030
Acknowledgements
The authors are grateful to the Ministry of Higher Education (MoHE) of Egypt for funding and supporting this work and the Egypt-Japan University of Science and Technology (E-JUST) for offering the tools and facilities required to perform this research work.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). This article was funded by the Ministry of Higher Education (MoHE) of Egypt.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study’s conception and design. Mohamed Nasser performed the model construction, data plotting, paper writing, discussion, and manuscript revising. However, Tamer F. Megahed conducted manuscript revision and supervision, whereas Shinichi Ookawara performed research supervision. Finally, Hamdy Hassan revised the manuscript, directed all the work, and was the main supervisor.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Ta Yeong Wu
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nasser, M., Megahed, T.F., Ookawara, S. et al. A review of water electrolysis–based systems for hydrogen production using hybrid/solar/wind energy systems. Environ Sci Pollut Res 29, 86994–87018 (2022). https://doi.org/10.1007/s11356-022-23323-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-022-23323-y