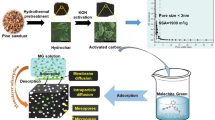
Abstract
In the present work, the fabrication of activated carbon (AC) from pomegranate husk (PHAC) by dual consecutive activation processes with ZnCl2 and NaOH as a chemical agent was studied. After that, the synthesized PHAC was used for adsorption of 4-chlorophenol (4CP) as a highly toxic compound for the human health and the environment. Different analytical techniques characterized the synthesized PHAC using ZnCl2/NaOH. The isotherms of N2 adsorption and desorption showed that total pore volume (Vtotal) and specific surface area (SBET) of PHAC were 0.404 cm3/g and 811.12 m2/g, respectively. The 4CP adsorption by PHAC studies revealed that the highest 4CP removal efficiency was 100% and obtained at 50, 100, and 150 mg/L of 4CP concentration with 2.5 g/L of PHAC. Based on the batch experiments, the highest 4CP removal was achieved at pH 6, 2.5 g/L of PHAC, and contact time of 60 min. The 4CP adsorption data of equilibrium and kinetics were successfully fitted to Langmuir’s isotherm and Avrami fractional order.















Similar content being viewed by others
References
Al-Degs YS, El-Barghouthi MI, El-Sheikh AH, Walker GM (2008) Effect of solution pH, ionic strength, and temperature on adsorption behavior of reactive dyes on activated carbon. Dyes Pigments 77:16–23
Babu KS, Reddy AR, Sujatha C, Reddy KV, Mallika A (2013) Synthesis and optical characterization of porous ZnO. J Adv Ceram 2:260–265
Badu Latip NM, Gopal K, Suwaibatu M, Hashim NM, Rahim NY, Raoov M, Yahaya N, Mohamad Zain NN (2020) Removal of 2, 4-dichlorophenol from wastewater by an efficient adsorbent of magnetic activated carbon. Sep Sci Technol 1–14
Bilgili MS (2006) Adsorption of 4-chlorophenol from aqueous solutions by xad-4 resin: isotherm, kinetic, and thermodynamic analysis. J Hazard Mater 137:157–164
Bilgili MS, Varank G, Sekman E, Top S, Özçimen D (2012) Modeling 4-chlorophenol removal from aqueous solutions by granular activated carbon. Environ Model Assess 17:289–300
Boudrahem F, Aissani-Benissad F, Aït-Amar H (2009) Batch sorption dynamics and equilibrium for the removal of lead ions from aqueous phase using activated carbon developed from coffee residue activated with zinc chloride. J Environ Manag 90:3031–3039
Chen C, Wang X (2007) Influence of pH, soil humic/fulvic acid, ionic strength and foreign ions on sorption of thorium (IV) onto γ-Al2O3. Appl Geochem 22:436–445
Fatehizadeh A, Zare MR, Van Ginkel SW, Taheri E, Amin MM, Rafiei N, Mahdavi M (2019) Methyl tertiary-butyl ether adsorption by bioactivated carbon from aqueous solution: kinetics, isotherm and artificial neural network modeling. Desalin Water Treat 154:254–267
Gratuito MKB, Panyathanmaporn T, Chumnanklang R-A, Sirinuntawittaya N, Dutta A (2008) Production of activated carbon from coconut shell: optimization using response surface methodology. Bioresour Technol 99:4887–4895
Gupta V, Nayak A (2012) Cadmium removal and recovery from aqueous solutions by novel adsorbents prepared from orange peel and Fe2O3 nanoparticles. Chem Eng J 180:81–90
Hadi S, Taheri E, Amin MM, Fatehizadeh A, Aminabhavi TM (2020) Synergistic degradation of 4-chlorophenol by persulfate and oxalic acid mixture with heterogeneous Fenton like system for wastewater treatment: adaptive neuro-fuzzy inference systems modeling. J Environ Manag 268:110678
Hameed B, Chin L, Rengaraj S (2008) Adsorption of 4-chlorophenol onto activated carbon prepared from rattan sawdust. Desalination 225:185–198
Jain S, Jayaram RV (2007) Adsorption of phenol and substituted chlorophenols from aqueous solution by activated carbon prepared from jackfruit (Artocarpus heterophyllus) peel-kinetics and equilibrium studies. Sep Sci Technol 42:2019–2032
Kilic M, Apaydin-Varol E, Pütün AE (2011) Adsorptive removal of phenol from aqueous solutions on activated carbon prepared from tobacco residues: equilibrium, kinetics and thermodynamics. J Hazard Mater 189:397–403
Kosmulski M (2009) Surface charging and points of zero charge, vol 145. CRC Press
Kuśmierek K, Świątkowski A, Kotkowski T, Cherbański R, Molga E (2020) Adsorption properties of activated tire pyrolysis chars for phenol and chlorophenols. Chem Eng Technol 43:770–780
Latinwo GK, Alade AO, Agarry SE, Dada EO (2019) Optimization of process parameters for the production of activated carbon from Delonix regia pod through chemical activation and carbonization process applied. J Environ Eng Sci 5:2075–2098
Li Y-H, Luan Z, Xiao X, Zhou X, Xu C, Wu D, Wei B (2003) Removal of Cu2+ ions from aqueous solutions by carbon nanotubes. Adsorpt Sci Technol 21:475–485
Li Y, Du Q, Wang X, Zhang P, Wang D, Wang Z, Xia Y (2010) Removal of lead from aqueous solution by activated carbon prepared from Enteromorpha prolifera by zinc chloride activation. J Hazard Mater 183:583–589
Lima DR et al (2019) Efficient acetaminophen removal from water and hospital effluents treatment by activated carbons derived from Brazil nutshells. Colloids Surf A Physicochem Eng Asp 583:123966
Maciá-Agulló J, Moore B, Cazorla-Amorós D, Linares-Solano A (2004) Activation of coal tar pitch carbon fibres: physical activation vs. chemical activation. Carbon 42:1367–1370
Monsalvo VM, Mohedano AF, Rodriguez JJ (2011) Activated carbons from sewage sludge: application to aqueous-phase adsorption of 4-chlorophenol. Desalination 277:377–382
Monsalvo VM, Mohedano AF, Rodriguez JJ (2012) Adsorption of 4-chlorophenol by inexpensive sewage sludge-based adsorbents. Chem Eng Res Des 90:1807–1814
Noorimotlagh Z, Shahriyar S, Darvishi Cheshmeh Soltani R, Tajik R (2016) Optimized adsorption of 4-chlorophenol onto activated carbon derived from milk vetch utilizing response surface methodology. Desalin Water Treat 57:14213–14226
Ribas MC, Adebayo MA, Prola LDT, Lima EC, Cataluña R, Feris LA, Puchana-Rosero MJ, Machado FM, Pavan FA, Calvete T (2014) Comparison of a homemade cocoa shell activated carbon with commercial activated carbon for the removal of reactive violet 5 dye from aqueous solutions. Chem Eng J 248:315–326
Sajjadi S-A, Meknati A, Lima EC, Dotto GL, Mendoza-Castillo DI, Anastopoulos I, Alakhras F, Unuabonah EI, Singh P, Hosseini-Bandegharaei A (2019) A novel route for preparation of chemically activated carbon from pistachio wood for highly efficient Pb (II) sorption. J Environ Manag 236:34–44
Sathishkumar M, Binupriya AR, Vijayaraghavan K, Yun SI (2007) Two and three-parameter isothermal modeling for liquid-phase sorption of procion blue H-B by inactive mycelial biomass of Panus fulvus. J Chem Technol Biotechnol 82:389–398
Shaarani F, Hameed B (2011) Ammonia-modified activated carbon for the adsorption of 2, 4-dichlorophenol. Chem Eng J 169:180–185
Srivastava S, Tyagi R, Pal N, Mohan D (1997) Process development for removal of substituted phenol by carbonaceous adsorbent obtained from fertilizer waste. J Environ Eng 123:842–851
Termoul M, Bestani B, Benderdouche N, Belhakem M, Naffrechoux E (2006) Removal of phenol and 4-chlorophenol from aqueous solutions by olive stone-based activated carbon. Adsorpt Sci Technol 24:375–388
Tseng R-L, Tseng S-K (2005) Pore structure and adsorption performance of the KOH-activated carbons prepared from corncob. J Colloid Interface Sci 287:428–437
Tseng R-L, Wu K-T, Wu F-C, Juang R-S (2010) Kinetic studies on the adsorption of phenol, 4-chlorophenol, and 2, 4-dichlorophenol from water using activated carbons. J Environ Manag 91:2208–2214
Umpierres CS, Thue PS, Lima EC, Reis GS, de Brum IAS, Alencar WS, Dias SLP, Dotto GL (2018) Microwave-activated carbons from tucumã (Astrocaryum aculeatum) seed for efficient removal of 2-nitrophenol from aqueous solutions. Environ Technol 39:1173–1187
Wang L, Zhang J, Zhao R, Li Y, Li C, Zhang C (2010) Adsorption of Pb (II) on activated carbon prepared from Polygonum orientale Linn.: kinetics, isotherms, pH, and ionic strength studies. Bioresour Technol 101:5808–5814
Wigmans T (1989) Industrial aspects of production and use of activated carbons. Carbon 27:13–22
Yadav S, Asthana A, Chakraborty R, Jain B, Singh AK, Carabineiro SAC, Susan MABH (2020) Cationic dye removal using novel magnetic/activated charcoal/β-cyclodextrin/alginate polymer nanocomposite. Nanomaterials 10:170
Funding
The current research was supported by Isfahan University of Medical Sciences (Grant No. 298169 and ethical code #IR.MUI.RESEARCH.REC.1398.636). E.C. Lima thanks to CNPq (303.622/2017-2) and FAPERGS (19/2551-0001865-7).
Author information
Authors and Affiliations
Contributions
Sousan Hadi: Investigation, Data curation, Writing (original draft preparation); Ensiyeh Taheri: Investigation, Data curation, Formal analysis, Writing (original draft preparation); Mohammad Mehdi Amin: Data curation, Writing (original draft preparation); Ali Fatehizadeh: Conceptualization, Supervision, Methodology, Writing (original draft preparation); Eder C. lima: Software, Writing (review and editing).
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Ethical approval
All procedures performed in this study were in accordance with the ethical standards of the Iranian national research committee and its later amendments or comparable ethical standards.
Consent to participate
The manuscript is an original work of all authors and all authors made a significant contribution to this study.
Consent to publish
The authors hereby consent to the publication of the work in Environmental Science and Pollution Research journal.
Additional information
Responsible Editor: Tito Roberto Cadaval Jr
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hadi, S., Taheri, E., Amin, M.M. et al. Fabrication of activated carbon from pomegranate husk by dual consecutive chemical activation for 4-chlorophenol adsorption. Environ Sci Pollut Res 28, 13919–13930 (2021). https://doi.org/10.1007/s11356-020-11624-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-11624-z