Abstract
The phenotypic diversity of ant workers plays a fundamental role in their biology. In this study, we asked if the body size variation of monomorphic workers of the ant Lasius niger (Formicidae) responds adaptively to metal pollution in a post-mining metal-polluted area. Nest samples of workers were collected along a pollution gradient to calculate the within-colony variance in body size (expressed as maximum head width, HW). The results showed that the body size variation of L. niger was unrelated to the pollution index but demonstrated considerable variation between colonies even within the same study site. We suggest that the differences in morphological diversity between the colonies of L. niger could be shaped by colony personality traits, i.e., by colony-specific foraging and/or the feeding efficiency of nursing workers. The study supports previous findings, showing that morphological traits in Lasius ants are weakly related to environmental metal pollution.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The morphological diversity of ant workers is believed to enhance colony performance. The association between the morphological variation of workers and colony fitness was confirmed mainly for ants that maintain morphologically distinguishable worker subcastes (Jaffe et al. 2007; Evison and Hughes 2011). Nevertheless, the vast majority of ants, even the most successful species, have monomorphic workers of limited size variation (Oster and Wilson 1978). Testing the adaptive meaning of body size variation in such species as well as its plasticity in response to environmental stress showed contradictory results (Beshers and Traniello 1994; Billick and Carter 2007; Modlmeier and Foitzik 2011; Colin et al. 2017). Some authors suggested that we need to improve our understanding of body size diversity among ants, by including research at the species and colony level (Gouws et al. 2011; Wills et al. 2018).
Worker body size and its distribution can be influenced by several potentially interacting factors acting within or outside the colony (reviewed in Wills et al. 2018). Genetic background, hormonal regulation, temperature, photoperiod, and predation pressure as well as competition with other ants are among the most important body size determinants of ants (Davidson 1978; Herbers 1980; Wheeler and Nijhout 1981; Bargum et al. 2004; Kipyatkov et al. 2004; Yang et al. 2004; Linksvayer 2006; Abril et al. 2010). For example, environment rich in food resources positively affects the body size of ant workers (Deslippe and Savolainen 1995). Additionally in ants, social organization also greatly contributes to the size variation. Typically, polymorphic ants produce small workers during early stages of colony founding, while the production of large workers grows as colony age increases (Hölldobler and Wilson 1990).
Ants are a common component of the fauna in metal polluted areas (Eeva et al. 2004; Grześ 2009; Belskaya et al. 2017; Frizzi et al. 2017). Body size variation in ants in such areas may be associated with metal pollution level for several reasons. First, pollution may influence the body size of ants, which relates to selection mechanisms leading to optimal ant size under such conditions. If natural selection acts on body size, its diversity should decrease proportionally to an increase in the local contamination level. Morphological diversity can be also affected indirectly, because the ants may be adaptively responding to reduced food resources; high diversity of workers could allow for more efficient foraging, enhancing colony fitness. Alternatively, pollution level and body size diversity may not be significantly correlated. It is likely that variation in body size is strongly colony-dependent due to the colony personality traits (sensu Wilson et al. 1994; Gosling 2001). It has been proven that ant colonies can differ considerably from one another in a consistent manner even within the same population in terms of their efficiency in foraging, brood feeding, or thermal regulation (DiRienzo and Dornhaus 2017; Pinter-Wollman 2012). Between-colony variation in body size diversity can be also driven by the genetic background of a colony. Polygyny and polyandry should significantly increase the size diversity of workers. One could also hypothesize that the body size variation of ants relates to the size of potential prey items. In consequence, the body size variation of workers may be better explained by the local diversity of prey around the nest than by the pollution level of the site. The high between-colony differences caused by the factors mentioned above may mask the positive or negative effect of metals on the body size diversity of workers.
In 2015, we published a study on the common garden ant Lasius niger (Grześ et al. 2015a) that addressed the within-nest distribution of body size in the same polluted area as that used in the present research. Apart from the skewness of the within-nest distribution curves, all other statistics, including the variation coefficient, did not significantly relate to the pollution level. In that study, we considered only 19 replicates; therefore, the results which were not significant were rather conservative and would constitute a type-II error. On the other hand, our most recent and on-going studies of ants originating from non-polluted areas showed that the trends in body size may be weak but still significant and likely of biological importance (Grześ et al. 2016). However, these results were detected when a big dataset was considered.
This study concerns morphological diversity of common garden ant Lasius niger along a metal-pollution gradient in a post-mining area, using a relatively high number of replicates, giving the chance to confirm the association between pollution and variation, if it truly exists. The study area is contaminated by high concentrations of zinc, cadmium, and lead. L. niger is very common in the investigated area. We expressed morphological diversity as the body size variations of the workers within the nests. We quantified their size by measuring head width, a standard measure of size in ants. We ask the question: “how flexible is body size diversity as a response to metal pollution”?
Materials and methods
Study area
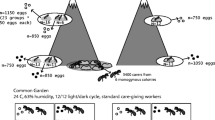
The study sites were located in the post-mining area of the Bolesław zinc-and-lead smelter in southern Poland. In a preliminary study, 10 study sites were established along the Zn and Cd soil pollution gradient covering formerly cultivated fields (eight sites) and industrial wastelands (two sites). The gradient extended from 0.7 to 10 km from the pollution source (Fig. 1). Most sites are currently covered by mixed ruderal-meadow-grasslands vegetation and have a xerothermic character with diversified types of plant communities, of which the Arrhenatheretum elatioris association was most frequent. Brown loamy or poorly loamy, neutral to base-rich soils, dominated the sites (Woch 2011). The total metal concentrations in the topsoil at the sites used in this study ranged from 158.36 to 16,984.90 mg/kg d.w. for Zn and from 3.36 to 63.86 mg/kg d.w. for Cd (Grześ et al. 2015a). Because ants in natural conditions are exposed to metal pollution via the trophic route (Maavara et al. 1994), we expressed the pollution index of each of the 10 study sites as the total concentration of Zn in a random sample of field-collected invertebrates that could potentially comprise the diet of L. niger. The transfer of metals from soil to invertebrates is well documented (Graff et al. 1997; Wilczek et al. 2005; van Straalen et al. 2005; Butovsky 2011; Ardestani and van Gestel 2013; Boshoff et al. 2013; Ding et al. 2013). The total concentration of Zn in invertebrates was used as a convenient pollution index in our previous studies performed in the Bolesław smelter area. The concentrations of both contaminants in invertebrates and in the soil were correlated (Grześ et al. 2015a; Grześ and Okrutniak 2016). The lowest and the highest Zn concentrations detected in the invertebrates were 134.86 and 1545.11 mg/kg d.w., respectively. Detailed metal concentrations in the soil and invertebrates, as well as a botanical description of all sites, are reported in Grześ et al. (2015a; see sites S5-S8, S10-S12, S14, S15 and S19).
Sampling
Lasius niger (L. 1758) is the dominant ant species inhabiting the investigated area; its relative abundance accounted for more than 70% of ants (Grześ 2009). This strictly monodomous species builds mineral nest mounds and is both carnivorous and aphidicolous. Nuptial flights take place from July to late August (Czechowski et al. 2012). Samples of L. niger workers were collected from mature colonies in the end of July 2016. In each of the 10 sites, 10 colonies were standardized for relatively large mound diameter for statistical purposes. The mound diameter ranged between 40 and 60 cm. In total, 100 colonies were investigated. A random sample of about 30 workers was collected using a plastic exhaustor just beyond the opening of the nest until the first sexuals were observed. The samples were stored at − 5 °C until measured.
Morphological measurements
The body size of ants was expressed as the maximum head width across the eyes (HW sensu Czechowski et al. 2012). Head width is commonly used as a convenient index of body size in a number ant species of the Formicinae subfamily (Yamauchi et al. 2001; Fjerdingstad 2005; Schwander et al. 2005; Aron et al. 2009). In total, 3000 workers were measured. Measurements were performed under a metallographic microscope Met-153 (Motic, China, × 100 magnification) with a digital camera (Huvitz, Korea). The head width of each ant was measured based on a digital photograph to the nearest 0.0001 mm using Panasis, ver. 2.4.2, Huvitz.
Statistical procedures
Two independent analyses were performed.
-
1.
We tested the relationship between pollution and body size diversity of L. niger using generalized regression model. We expressed body size diversity as intra-colony variances in head width (HW). Thus, we obtained one measure for each colony. Then, we regressed the calculated variances against metal pollution index, i.e., the total concentration of Zn in a random sample of field-collected invertebrates that could potentially comprise the diet of L. niger. The distribution of the calculated variances was found to be right-skewed; therefore, we used generalized regression model (GLMs) with gamma distributed dependent variable and identity link function (Welder 1972). Model was fitted using the STATISTICA program (StatSoft Inc., 2001).
As ants are social insects living in colonies, we assumed that each colony should be treated as an independent replicate. Because 10 colonies were chosen at each site, which results in a special dependence of colonies, the colony may seem a pseudoreplication for each study site. To avoid this problem, we followed the advice of Davies and Gray (2015) by limiting our conclusions to the specific gradient at a specific site and not to the metal pollution per se. Therefore, we are aware that our final findings are rather more of a hypothesis for future studies than irrefutable evidence.
-
2.
In order to analyze which factor—the site of origin or the colony from which ants were sampled—contributes the most variance in individual body size (head width), the variance components analysis was performed. Regarding the hierarchical nature of the data, the factor “colony” nested in the random factor “site” was incorporated in the model as a categorical random factor. The output of the analysis is the percentage of the total variance represented by each factor. The analysis was validated by testing whether the residuals met the assumptions of normality using the Shapiro-Wilk’s test using Statgraphics Centurion XV.
Results
No significant correlation was found between the pollution index and intra-colony variance in body size (N = 100, χ2 = 1.06, P = 0.30, Fig. 2), indicating that metal pollution does do not diminish nor increase the body size variation of the investigated ant. The distribution of variances is right-skewed (standardized skewness = 4.7, Fig. 3), indicating that ants of several nests are highly variable irrespective of the metal pollution index of their origin. The contribution of the factors colony and site represents 20.8% and 6.8% of the total variation in body size, respectively. The 72.4% of the total variance is unexplained by the above factors (error factor).
The relationship between the variance in the size of head width in different colonies and the pollution index of the site. The pollution index is expressed as the total Zn concentration (mg/kg d.w.) in a random sample of small invertebrates collected at each of 10 study sites. Each point represents the variance of one colony (N = 100)
Discussion
The study was done to assess the body size diversity in the common garden ant at 10 independent sites forming a well-known metal pollution gradient. The body size variance calculated for each of the 100 nests turned out to be not associated with the pollution index of the site of origin, suggesting a relatively low flexibility of body size variation in the response to environmental metal pollution. We indicated that more variance was attributable to the differences between the colonies than to the differences between the sites with different pollution values. Because body size diversity did not increase or decrease with increasing pollution, it seems that L. niger workers do not adapt to metal pollution by extending or narrowing body size range. We also found no support for the hypothesis that metal pollution selects the ants for the particular body size. Although the toxic effects of trace metal pollution on ants are recognized (e.g., Sorvari et al. 2007 for disturbance of ant immune response system), evidence for the adaptation to environmental metal pollution in an evolutionary sense, or at least for increased metal tolerance, is very limited (Grześ 2010; Grześ and Okrutniak 2016). Proof of increased Zn tolerance has been demonstrated for the adult workers of Myrmica rubra, showing that workers collected in polluted sites were less metal sensitive than those collected in control areas (Grześ 2010).
The within-colony body size variances do not correlate with the pollution index, but the values of these variances are diversified between the colonies. This is consistent with the variance components analysis showing that a greater amount of variance in body size is due to the differences between the colonies than to the differences between the sites of origin. This means that colony-dependent factors, presumably due to genetic background or colony personality traits, are more important in shaping variation in body size of the investigated species than site-specific external environmental conditions. These factors contribute to the between-colony differences in body size variation so that the effect of pollution is not statistically detectable although we used relatively big data set. Genetic diversity at the colony level of many ant species increases body size diversity (Fjerdingstad and Crozier 2006). The investigated species has one reproductive female in the nest, but the number of its sexual partners may differ between nests, providing a variable number of parental lines of the offspring (Fjerdingstad and Keller 2004). This may at least partially explain the genetic basis for the observed between-colony differences in body size diversity. We suggest that observed intra-colony variance in the body size could be treated as a secondary effect of colony personality.
It cannot be excluded that other non-social environmental factors unrelated to pollution but acting locally could also contribute to the observed high variation in the body size between the colonies. Assuming that the study sites are diversified in the respect of microhabitats, the colonies may be subjected to different temperature and humidity. It was found the nest temperature effects of worker size in Formica aquilonia (Sorvari and Hakkarainen 2009). It is also possible that the colony-specific body size diversity is driven by the adjustment of worker size and the size of potential preys around the nest. However, testing this hypothesis would require detailed data on local food variation and their contribution to the diet of ants of each colony.
The presented results are in agreement with those obtained in our previous studies on Lasius ants indicating that their morphological characteristics are usually unrelated to the pollution level. Based on correlative studies, we found no association between average body size in mature or immature colonies of L. niger (Grześ et al. 2015a, 2015b). Similarly, the asymmetry of the eyes of Lasius flavus was not associated with pollution, suggesting the ability of ants to maintain a stable and repetitive trajectory of eye development during the larval stage (Grześ et al. 2015c). Comparing the studies of other authors, ants seem to be much less sensitive to pollution-induced morphological changes than carabids in metal-polluted sites (Maryański et al. 2002; Łagisz 2008) as well as in urbanized areas (Weller and Ganzhorn 2004; Sukhodolskaya 2013). Body size decrease in carabids might be explained by the limited resource availability during larval stage development or by the energy reallocation from growth to detoxification (Łagisz 2008). The environmentally induced changes in morphological parameters were postulated to be permissible indicators in metal pollution risk assessment (Skaldina and Sorvari 2017). However, as the morphological diversity of L. niger workers was not associated with the pollution index at the site of their origin, the present study gives no good reasons for recommending size variation of this species as an efficient metal bioindicator.
To summarize, variation in the body size of the monomorphic ant Lasius niger was unrelated to the pollution index along the investigated pollution gradient, but demonstrated considerable variation between colonies even within the same study site. It seems that colony-dependent factors are more important in shaping variation in the body size of the investigated species than the pollution level. The study supports previous findings showing that morphological traits in ants are weakly related to environmental metal pollution.
References
Abril S, Oliveras J, Gomez C (2010) Effect of temperature on the development and survival of the Argentine ant, Linepithema humile. J Insect Sci 10(97). https://doi.org/10.1673/031.010.9701
Ardestani MM, van Gestel CAM (2013) Dynamic bioavailability of copper in soil estimated by uptake and elimination kinetics in the springtail Folsomia candida. Ecotoxicology 22:308–318. https://doi.org/10.1007/s10646-012-1027-8
Aron S, Steinhauer N, Fournier D (2009) Influence of queen phenotype, investment and maternity apportionment on the outcome of fights in cooperative foundations of the ant Lasius niger. Anim Behav 77:1067–1074. https://doi.org/10.1016/j.anbehav.2009.01.009
Bargum K, Boomsma JJ, Sundstrom L (2004) A genetic component to size in queens of the ant, Formica truncorum. Behav Ecol Sociobiol 57:9–16. https://doi.org/10.1007/s00265-004-0836-z
Belskaya E, Gilev A, Belskii E (2017) Ant (Hymenoptera, Formicidae) diversity along a pollution gradient near the Middle Ural Copper Smelter, Russia. Environ Sci Pollut R 24:10768–10777. https://doi.org/10.1007/s11356-017-8736-8
Beshers SN, Traniello JFA (1994) The adaptiveness of worker demography in the attine ant Trachymyrmex septentrionalis. Ecology 75:763–775. https://doi.org/10.2307/1941733
Billick I, Carter C (2007) Testing the importance of the distribution of worker sizes to colony performance in the ant species Formica obscuripes Forel. Insect Soc 54:113–117. https://doi.org/10.1007/s00040-007-0918-9
Boshoff M, Jordaens K, Backeljau T, Lettens S, Tack F, Vandecasteele B, De Jonge M, Bervoets L (2013) Organ- and species-specific accumulation of metals in two land snail species (Gastropoda, Pulmonata). Sci Total Environ 449:470–481. https://doi.org/10.1016/j.scitotenv.2013.02.003
Butovsky RO (2011) Heavy metals in carabids (Coleoptera, Carabidae). ZooKeys 100:215–222. https://doi.org/10.3897/zookeys.100.1529
Colin T, Doums C, Peronnet R, Molet M (2017) Decreasing worker size diversity does not affect colony performance during laboratory challenges in the ant Temnothorax nylanderi. Behav Ecol Sociobiol 71(92). https://doi.org/10.1007/s00265-017-2322-4
Czechowski W, Radchenko A, Czechowska W, Vepsäläinen K (2012) The ants of Poland with reference to the myrmecofauna of Europe. Museum and Institute of Zoology of the Polish Academy of Sciences and Natura optima dux Foundation, Warszawa
Davidson DE (1978) Size variability in the worker caste of a social insect (Vermessor pergandei Mayr) as a function of the competitive environment. Am Nat 112:523–532
Davies GM, Gray A (2015) Don't let spurious accusations of pseudoreplication limit our ability to learn from natural experiments (and other messy kinds of ecological monitoring). Ecol Evol 5:5295–5304. https://doi.org/10.1002/ece3.1782
Deslippe RJ, Savolainen R (1995) Sex investment in a social insect—the proximate role of food. Ecology 76:375–382. https://doi.org/10.2307/1941196
Ding P, Zhuang P, Li Z, Xia HP, Lu HP (2013) Accumulation and detoxification of cadmium by larvae of Prodenia litura (Lepidoptera: Noctuidae) feeding on Cd-enriched amaranth leaves. Chemosphere 91:28–34. https://doi.org/10.1016/j.chemosphere.2012.11.038
DiRienzo N, Dornhaus A (2017) Temnothorax rugatulus ant colonies consistently vary in nest structure across time and context. PLoS One 12(10). https://doi.org/10.1371/journal.pone.0177598
Eeva T, Sorvari J, Kolvunen V (2004) Effects of heavy metal pollution on red wood ant (Formica s. str.) populations. Environ Pollut 132:533–539. https://doi.org/10.1016/j.envpol.2004.05.004
Evison SEF, Hughes WOH (2011) Genetic caste polymorphism and the evolution of polyandry in Atta leaf-cutting ants. Naturwissenschaften 98:643–649. https://doi.org/10.1007/s00114-011-0810-3
Fjerdingstad EJ (2005) Control of body size of Lasius niger ant sexuals—worker interests, genes and environment. Mol Ecol 14:3123–3132. https://doi.org/10.1111/j.1365-294X.2005.02648.x
Fjerdingstad EJ, Crozier RH (2006) The evolution of worker caste diversity in social insects. Am Nat 167:390–400. https://doi.org/10.1086/499545
Fjerdingstad EJ, Keller L (2004) Relationships between phenotype, mating behavior, and fitness of queens in the ant Lasius niger. Evolution 58:1056–1063. https://doi.org/10.1111/j.0014-3820.2004.tb00439.x
Frizzi F, Masoni A, Celikkol M, Palchetti E, Ciofi C, Chelazzi G, Santini G (2017) Serpentine soils affect heavy metal tolerance but not genetic diversity in a common Mediterranean ant. Chemosphere 180:326–334. https://doi.org/10.1016/j.chemosphere.2017.04.026
Gosling SD (2001) From mice to men: what can we learn about personality from animal research? Psychol Bull 127:45–86. https://doi.org/10.1037/0033-2909.127.1.45
Gouws EJ, Gaston KJ, Chown SL (2011) Intraspecific body size frequency distributions of insects. PLoS One 6:e16606. https://doi.org/10.1371/journal.pone.0016606
Graff S, Berkus M, Alberti G, Kohler HR (1997) Metal accumulation strategies in saprophagous and phytophagous soil invertebrates: a quantitative comparison. Biometals 10:45–53. https://doi.org/10.1023/a:1018366703974
Grześ IM (2009) Ant species richness and evenness increase along a metal pollution gradient in the Boleslaw zinc smelter area. Pedobiologia 53:65–73. https://doi.org/10.1016/j.pedobi.2009.03.002
Grześ IM (2010) Zinc tolerance in the ant species Myrmica rubra originating from a metal pollution gradient. Eur J Soil Biol 46:87–90. https://doi.org/10.1016/j.ejsobi.2009.11.005
Grześ IM, Okrutniak M (2016) Pre-adaptive cadmium tolerance in the black garden ant. Chemosphere 148:316–321. https://doi.org/10.1016/j.chemosphere.2016.01.059
Grześ IM, Okrutniak M, Woch MW (2015a) Monomorphic ants undergo within-colony morphological changes along the metal-pollution gradient. Environ Sci Pollut R 22:6126–6134. https://doi.org/10.1007/s11356-014-3808-5
Grześ IM, Okrutniak M, Antosik G (2015b) Body size of the monomorphic ant Lasius niger: Young colonies along a metal-pollution gradient. Psyche: A Journal of Entomology 2015:Article ID 873415, 5 pages. https://doi.org/10.1155/2015/873415
Grześ IM, Okrutniak M, Szpila P (2015c) Fluctuating asymmetry of the yellow meadow ant along a metal-pollution gradient. Pedobiologia 58:195–200. https://doi.org/10.1016/j.pedobi.2015.11.001
Grześ IM, Okrutniak M, Grzegorzek J (2016) The size-dependent division of labour in monomorphic ant Lasius niger. Eur J Soil Biol 77:1–3. https://doi.org/10.1016/j.ejsobi.2016.08.006
Herbers JM (1980) On caste ratios in ant colonies—population responses to changing environments. Evolution 34:575–585. https://doi.org/10.2307/2408225
Hölldobler B, Wilson EO (1990) The ants. Belknap Press, Cambridge
Jaffe R, Kronauer DJC, Kraus FB, Boomsma JJ, Moritz RFA (2007) Worker caste determination in the army ant Eciton burchellii. Biol Lett 3:513–516. https://doi.org/10.1098/rsbl.2007.0257
Kipyatkov VE, Lopatina EB, Imamgaliev AA, Shirokova LA (2004) Effect of temperature on rearing of the first brood by the founder females of the ant Lasius niger (Hymenoptera, Formicidae): latitude-dependent variability of the response norm. J Evol Biochem Physiol 40:165–175. https://doi.org/10.1023/b:joey.0000033808.45455.75
Łagisz M (2008) Changes in morphology of the ground beetle Pterostichus oblongopunctatus F. (Coleoptera; Carabidae) from vicinities of a zinc and lead smelter. Environ Toxicol Chem 27:1744–1747. https://doi.org/10.1897/07-661.1
Linksvayer TA (2006) Direct, maternal, and sibsocial genetic effects on individual and colony traits in an ant. Evolution 60:2552–2561. https://doi.org/10.1554/06-011.1
Maavara V, Martin A-J, Oja A, Nuorteva P (1994) Sampling of different social categories of red wood ants (Formica s. str.) for biomonitoring. In: Berndt M (ed) Environmental sampling for trace analysis. VCH Verlagsgesellschaft mbH, Weinheim, pp 465–489
Maryański M, Kramarz P, Laskowski R, Niklinska M (2002) Decreased energetic reserves, morphological changes and accumulation of metals in carabid beetles (Poecilus cupreus L.) exposed to zinc- or cadmium-contaminated food. Ecotoxicology 11:127–139. https://doi.org/10.1023/a:1014425113481
Modlmeier AP, Foitzik S (2011) Productivity increases with variation in aggression among group members in Temnothorax ants. Behav Ecol 22:1026–1032. https://doi.org/10.1093/beheco/arr086
Oster GF, Wilson EO (1978) Caste and ecology in the social insects. Princeton University Press, Princeton
Pinter-Wollman N (2012) Personality in social insects: how does worker personality determine colony personality? Curr Zool 58:580–588. https://doi.org/10.1093/czoolo/58.4.580
Schwander T, Rosset H, Chapuisat M (2005) Division of labour and worker size polymorphism in ant colonies: the impact of social and genetic factors. Behav Ecol Sociobiol 59:215–221. https://doi.org/10.1007/s00265-005-0027-6
Skaldina O, Sorvari J (2017) Biomarkers of ecotoxicological effects in social insects. In: Kesari KK (ed) Perspectives in environmental toxicology. Environmental Science and Engineering. Springer, Cham, pp 203–214
Sorvari J, Hakkarainen H (2009) Forest clear-cutting causes small workers in the polydomous wood ant Formica aquilonia. Ann Zool Fenn 46:431–438. https://doi.org/10.5735/086.046.0604
Sorvari J, Rantala LM, Rantala MJ, Hakkarainen H, Eeva T (2007) Heavy metal pollution disturbs immune response in wild ant populations. Environ Pollut 145:324–328. https://doi.org/10.1016/j.envpol.2006.03.004
van Straalen NM, Donker MH, Vijver MG, van Gestel CAM (2005) Bioavailability of contaminants estimated from uptake rates into soil invertebrates. Environ Pollut 136:409–417. https://doi.org/10.1016/j.envpol.2005.01.019
Sukhodolskaya R (2013) Intraspecific body size variation in ground beetles (Coleoptera, Carabidae) in urban - suburban - rural - natural gradient. Acta Biol Univ Daugavpiliensis 13:121–128
Welder JA, Wedderburn RWM (1972) Generalized linear models. J R Statist Soc A 135:370–384. https://doi.org/10.2307/2344614
Weller B, Ganzhorn JU (2004) Carabid beetle community composition, body size, and fluctuating asymmetry along an urban-rural gradient. Basic Appl Ecol 5:193–201. https://doi.org/10.1078/1439-1791-00220
Wheeler DE, Nijhout HF (1981) Soldier determination in ants: new role for juvenile hormone. Science 213:361–363. https://doi.org/10.1126/science.213.4505.361
Wilczek G, Babczynska A, Majkus Z (2005) Body burdens of metals in spiders from the Lidice coal dump near Ostrava (Czech Republic). Biol Section Zool 60:599–605
Wills BD, Powell S, Rivera MD, Suarez AV (2018) Correlates and consequences of worker polymorphism in ants. Annu Rev Entomol 63:575–598. https://doi.org/10.1146/annurev-ento-020117-043357
Wilson DS, Clark AB, Coleman K, Dearstyne T (1994) Shyness and boldness in humans and other animals. Trends Ecol Evol 9:442–446. https://doi.org/10.1016/0169-5347(94)90134-1
Woch MW (2011) Xerothermic vegetation of fallow lands in western Małopolska. Annales Universitatis Mariae Curie-Skłodowska Lublin-Polonia Sectio C 66:105–120. https://doi.org/10.2478/v10067-011-0022-4
Yamauchi K, Yoshida T, Ogawa T, Itoh S, Ogawa Y, Jimbo S, Imai HT (2001) Spermatogenesis of diploid males in the formicine ant, Lasius sakagamii. Insect Soc 48:28–32. https://doi.org/10.1007/pl00001741
Yang AS, Martin CH, Nijhout HF (2004) Geographic variation of caste structure among ant populations. Curr Biol 14:514–519. https://doi.org/10.1016/j.cub.2004.03.005
Funding
This research was financed by the Ministry of Science and Higher Education of the Republic of Poland.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Responsible editor: Giovanni Benelli
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Grześ, I.M., Okrutniak, M., Gorzałczany, M. et al. Body size variation of the ant Lasius niger along a metal pollution gradient. Environ Sci Pollut Res 26, 17858–17864 (2019). https://doi.org/10.1007/s11356-019-04811-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-019-04811-0